 Open Access
Open Access
REVIEW
The Electrophysiology of Semantic Processing in Individuals with Autism Spectrum Disorder: A Meta-Analysis
1 The National Clinical Research Center for Mental Disorder & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
2 Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
* Corresponding Author: Zhanjiang Li. Email:
(This article belongs to the Special Issue: Mental Health and Social Development)
International Journal of Mental Health Promotion 2023, 25(10), 1067-1079. https://doi.org/10.32604/ijmhp.2023.041430
Received 22 April 2023; Accepted 20 June 2023; Issue published 03 November 2023
Abstract
Language difficulties vary widely among people with autism spectrum disorder (ASD). However, the semantic processing of autistic person and its underlying electrophysiological mechanism are still unclear. This meta-analysis aimed to explore the disturbance of semantic processing in patients with ASD. PubMed, Web of Science, and Embase were searched for event-related potential (ERP) studies on semantic processing in autistic people published in English before September 01, 2022. Pooled estimates were calculated by fixed-effects or random-effects models according to the heterogeneity using Comprehensive Meta-Analysis 2.0. The potential moderators were explored by meta-regression and subgroup analysis. This meta-analysis has been registered at the Prospero International Prospective Register of Systematic Reviews (no. CRD 42021265852). A total of 14 articles and 18 studies, including 254 autistic people and 262 neurodevelopmental people were included in this meta-analysis. Compared to the comparison group, autistic people showed an overall reduced N400 amplitude (Hedges’ g = 0.350, p < 0.001) in response to linguistic stimuli instead of non-linguistic stimuli. The N400 amplitude was affected by verbal intelligence and gender. The reduced overall N400 amplitude in autistic people under linguistic stimuli suggests a linguistic-specific deficit in semantic processing in individuals of autism. The decrease of N400 amplitude might be a promising indication of the pool language capacity of autism.Keywords
Autism spectrum disorder (ASD) is a range of neurodevelopmental conditions characterized by impairments in social interaction as well as restricted/repetitive behavior and interests [1]. Although challenges with language is no longer a core symptom of ASD, it is still more extensive than assumed in autistic individuals. In addition, there is marked variability in language proficiency of ASD patients, ranging from silent to verbally fluent. Compared to language production, language comprehension difficulties may be more severe and may serve as an early sign of ASD [2,3]. Semantic processing, which means understanding and categorizing the meaning of stimulus, is considered to play a vital role in language comprehension [4]. Some studies suggested that semantics might provide a foundational language skill for social interactions and social skills in children with autism [5]. Event-related potential (ERP) with millisecond resolution is an efficient and sensitive technique for characterizing subtle differences in semantic processing [6]. As reflecting the neural mechanism of language comprehension, the N400 and the P600 are the most important ERP components in language progressing [4,7].
The N400 component is one of the most powerful tools used for semantic processing and integration research. It was first noted by Kutas and Hillyard as a language measure [8]. Although it is a negative peaking over the central-posterior electrode sites, it need not be negative in absolute terms [9]. The N400 effect refers to a difference ERP created via subtraction of a congruent condition from an incongruent one [10] and reflects the activation of related semantic networks [11]. The N400 response is often elicited by two paradigms. The semantic-anomaly paradigm generates a narrative with contextually congruent or incongruent sentence-final words. The amplitude of the N400 is greater for the incongruent condition because integration is difficult in incongruent context [12]. The semantic-priming paradigm provides a related or unrelated word before a target word and the priming word is considered to be the context for the target word to be integrated [13]. The decrease in the amplitude of N400 reflects the response to the target word, which is semantically related to the previous words [14]. Both of the two paradigms reflect the process of semantic integration of the critical word with the working context. Another theory supported that the N400 response reflects facilitated activation of the long-term memory as N400 is sensitive to word frequency [15]. Generally speaking, high-frequency and early-acquired words result in shorter N400 latency and a smaller N400 amplitude relative to less frequent words as they are easier to access from memory [16]. In addition, other factors that may alter the N400 amplitude include language proficiency and modalities [9]. Now, the function of N400 has been expanded to reflect meaning processing more broadly. Nonlinguistic context such as picture sequences was found to elicit N400 effect. However, the N400 effects elicited by nonlinguistic context is more frontally-distributed in scalp topography [17].
So far, there are considerable variabilities in N400 response among individuals with autism. A few studies have concluded that autistic people exhibit decreased or disappeared N400 effect, suggesting destruction of the neural response in language processing [5,18]. Meanwhile, in some studies, the N400 effect is typical in autistic people, with more significant negativity elicited by the incongruent stimulation [19–21]. By comparing the N400 component between autistic and non-autistic group, some studies found that autistic people showed abnormal N400 response [22]. Previous studies have confirmed that N400 could also be elicited by paradigms represented with the nonverbal stimuli such as visual narratives and environmentally sound. Some studies suggested that autistic people experience deficits in the semantic processing of language, but the semantic processing of non-verbal stimuli is intact [23,24]. While some literature suggested that autistic person showed a potential benefit of using verbal material but a medium difficulty with visual long-term memory [25].
P600, a positive deflection in centro-parietal is assumed to be a family member of the late positive components (LPC). P600 is considered as another important language-related ERPs and was first characterized in the context of syntactic processing [26]. Recent studies suggested that P600 might reflect more general conflict monitoring mechanisms rather than purely syntactic processes [27]. Previous studies have not yet determined whether autistic individuals present abnormal P600 under semantic or syntactic violation conditions.
The inconsistent results of language related ERPs in ASD may be derived from the heterogeneity generated by individual differences, modalities, and stimulus materials. The individual heterogeneity is assumed to originate from age (children/adolescents or adults), diagnosis (autism or Asperger syndrome), and cognitive ability. For the heterogeneity in modalities, the N400 effect was elicited either from unimodal tasks (visual or auditory) or from cross-modal paradigms given the high multisensory nature of language cues. For the heterogeneity in materials, the N400 effect was obtained from lexical associative priming or sentence-level congruity.
To date, evidence assessing the language-related ERPs in ASD is lacking, especially when compared the language-related ERPs among different experimental stimulus and paradigms. This meta-analysis therefore updates the current knowledge on semantic processing disturbance in patients with autism. The aims of this study were (1) to examine the neural characteristics of semantic processing in autistic people; (2) to explore the factors affecting semantic processing ERP feature in patients with ASD.
This review was registered at the Prospero International Prospective Register of Systematic Reviews (PROSPERO no. CRD 42021265852) and followed guidance of conducting and reporting systematic reviews from the Cochrane handbook and the PRISMA checklist assessed by the editorial team of PRISMA.
The articles were searched before September 01 2022 in PubMed, Web of science, and Embase. The search terms we used were MeSH phrases and text words related to autism spectrum disorder (“Autistic disorder” or “Asperger Syndrome” or “Autistic Spectrum Disorder” or “Disorder, Autistic Spectrum”), semantics (“semantic” or “language” or “linguistics”) and Event Related Potential (“N400” or “P600” or “Potential, Event-Related” or “Potential, Event Related”). The combination of search terms was listed in Suppl. Table S1.
Publications were only included in the analysis if: (1) studies published in English with full text available; (2) the study included between-group comparison with autistic and non-autsitic peers; (3) N400 or P600 component was measured to assess the semantic processing; (4) the incongruent condition minus the congruent condition or the unrelated condition minus the related condition was used to represent the different wave; (5) the time windows of N400 and P600 were 200–600 and 600–1000 ms, respectively.
Two reviewers (Danfeng Yuan and Xiangyun Yang) independently extracted and checked the data. Any disagreement was resolved through discussion until a consensus was reached with the third reviewer (Zhanjiang Li). We extracted data from selected studies regarding the number of participants, mean age, diagnosis, verbal Intelligence Quotient (IQ), non-verbal IQ, full-scale IQ, receptive language ability, stimulus characteristics, Autism Quotient scores (AQ) as well as mean and standard deviation or F-value of effect sizes in each group. Five effect sizes were computed to examine the pattern of semantic processing in autistic individuals: N400 amplitude, N400 effect (the difference in the N400 amplitude between congruent and incongruent conditions), N400 amplitudes for congruent conditions, N400 amplitudes for incongruent conditions, and P600 amplitude. As words generally elicited a more centro-parietal N400 and pictures elicited a more frontally distributed N400, we mainly extracted N400 amplitude elicited by linguistic stimulus at centro-parietal sites and extracted N400 amplitude elicited by non-linguistic stimulus at frontal sites. A total of 14 articles were retained for the current meta-analysis. Among these, one study contained participants with two different age group and three studies had two different N400 time windows, each experiment was taken as an independent study, making a total of 18 datasets for meta-analysis.
We divided N400 data into two categories according to the “stimulus types”: linguistic and non-linguistic stimulus. Furthermore, paradigms of the semantic process were classified into three categories: semantic anomalies, semantic priming, and in-category and out-of-category words. The “N400 effect” and the “P600 effect” were defined as the amplitude difference between related and unrelated conditions or congruent and incongruent conditions. The statistical analysis was performed by Comprehensive Meta-Analysis 2.0 (CMA-Version 2 professional, Biostat Inc., Englewood, USA).
Hedge’s g was calculated to correct for potential overestimation of the true effect in small study sample [28]. Hedge’s g and 95% confidence interval (95% CI) were presented as effect sizes by the mean amplitude differences between the autistic and comparison groups, divided by the pooled standard deviations. When means and standard deviations were unavailable, the effect size was computed from F value or mean change scores with t or p-value within groups.
Heterogeneity between studies was examined by Cochran’s Q and I2 tests, which helps to evaluate the consistency across studies [29]. The pooled effect size were analyzed with a random-effects model when the heterogeneity was nonnegligible (I2 > 50%) or a fixed-effects model when the heterogeneity is acceptable (I2≤ 50%).
The subgroup analysis and meta-regression were employed to explore potential sources of heterogeneity. The subgroup analysis categories were age, diagnosis, paradigm, modality, verbal level of the stimulus, and electrode site. The age was classified into two groups (children and adolescent, adults). The diagnosis was based on the DSM-IV and classified into Asperger syndrome and autism. As the N400 response can be elicited by several kinds of paradigms, we divided the paradigms into three groups (semantic priming, semantic anomalies, in-category and out-of-category words). Since the N400s are modality-dependent, we classified the studied according to modalities (auditory stimulus, visual stimulus, audiovisual stimulus). The studies’ characteristics were divided into three groups based on the electrode site (Fz, Cz, Pz). Furthermore, according to the processing levels of the stimulus, the studies were classified into two groups (word level, sentence level). The meta-regression was performed with gender, verbal IQ, performance/non-verbal IQ, full-scale IQ, receptive language proficiency, Autism Quotient scores, and trials of each condition.
A funnel plot was conducted to detect whether the included studies had publication bias. The funnel plot was examined visually when the pool studies reached 10 [30]. A more symmetrical funnel shape indicates less bias between studies. To quantify the extent of asymmetry of the plot, we also applied the Egger test to check the publication bias [31].
A total of 157 primary literature related to search protocol were discovered: 42 from PubMed, 81 from Web of Science and 34 from Embase. After selecting titles and abstracts, the full texts of 30 were considered potentially relevant. In the end, 14 articles were included in the meta-analysis after excluding 16 articles for the following reason: data no available, no case-control study, and no semantically related condition. The flowchart of study selection and inclusion is shown in Fig. 1.
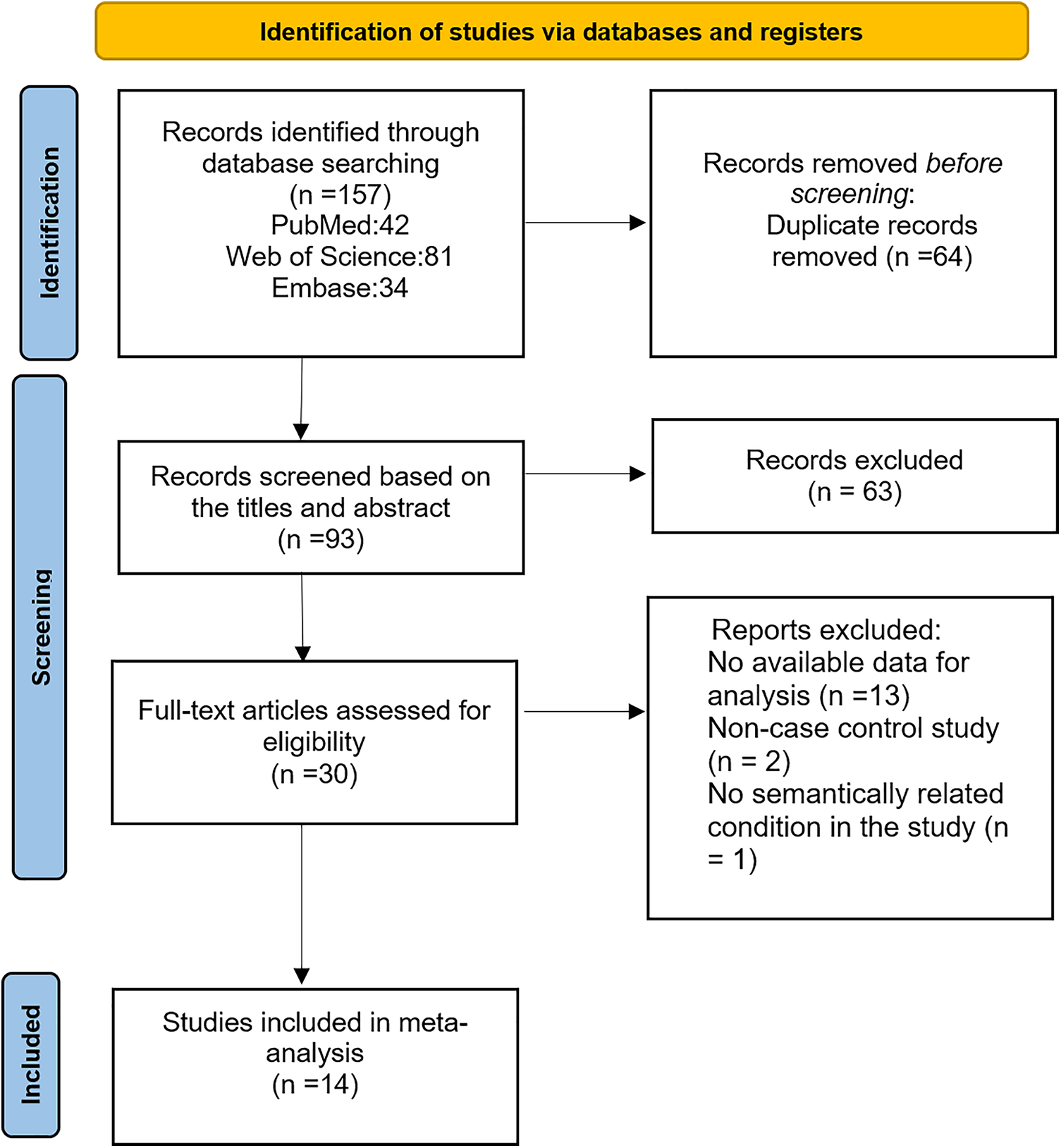
Figure 1: Flowchart of the study’s inclusion and exclusion criteria.
The characteristics of the included studies are listed in Table 1. All of the studies were conducted between 2005 and 2022. In total, this meta-analysis pooled results from 254 autistic people and 262 neurotypical controls, in which half of the subjects were children and adolescents and half were adults. Most of the studies included in the meta-analysis enrolled autistic people without intellectual disability. Two studies recruited subjects with Asperger syndrome. In one study, autistic children with minimally verbal were recruited. There are two kinds of stimuli in the studies including verbal materials and visual materials. According to the “stimulus characteristics”, articles were divided into two categories (linguistic and non-linguistic stimuli).
ERP component elicited by linguistic stimulus
In 14 articles and 18 studies, the N400 and P600 were elicited by linguistic stimuli [18,21,22,32–42]. Among the linguistic stimuli, some were presented with sentence in the semantic anomalies paradigm; the others were presented with words in the semantic priming paradigm or in-category and out-of-category words paradigm. Five effect sizes were computed to examine the N400 and P600 responses under the linguistic stimulus, including N400 amplitude, N400 different wave, N400 amplitude in congruent condition, N400 amplitude in incongruent condition, and P600 amplitude.
The N400 amplitudes difference under linguistic stimulus
Thirteen articles and 17 studies compared N400 amplitude differences elicited by linguistic stimuli between autistic and nonautistic group [18,21,22,32–41]. The sample size of the two groups were 218 and 237, respectively. As the heterogeneity was acceptable, a fixed effect model was used for analysis (I2= 0.00%, Q = 12.614). The analysis revealed a significantly reduced overall N400 amplitude in response to linguistic stimuli in the autistic group (Hedges’ g = 0.350, 95% CI ranged from 0.187 to 0.498, p < 0.001, Fig. 2). Further analysis with potential moderators was evaluated.
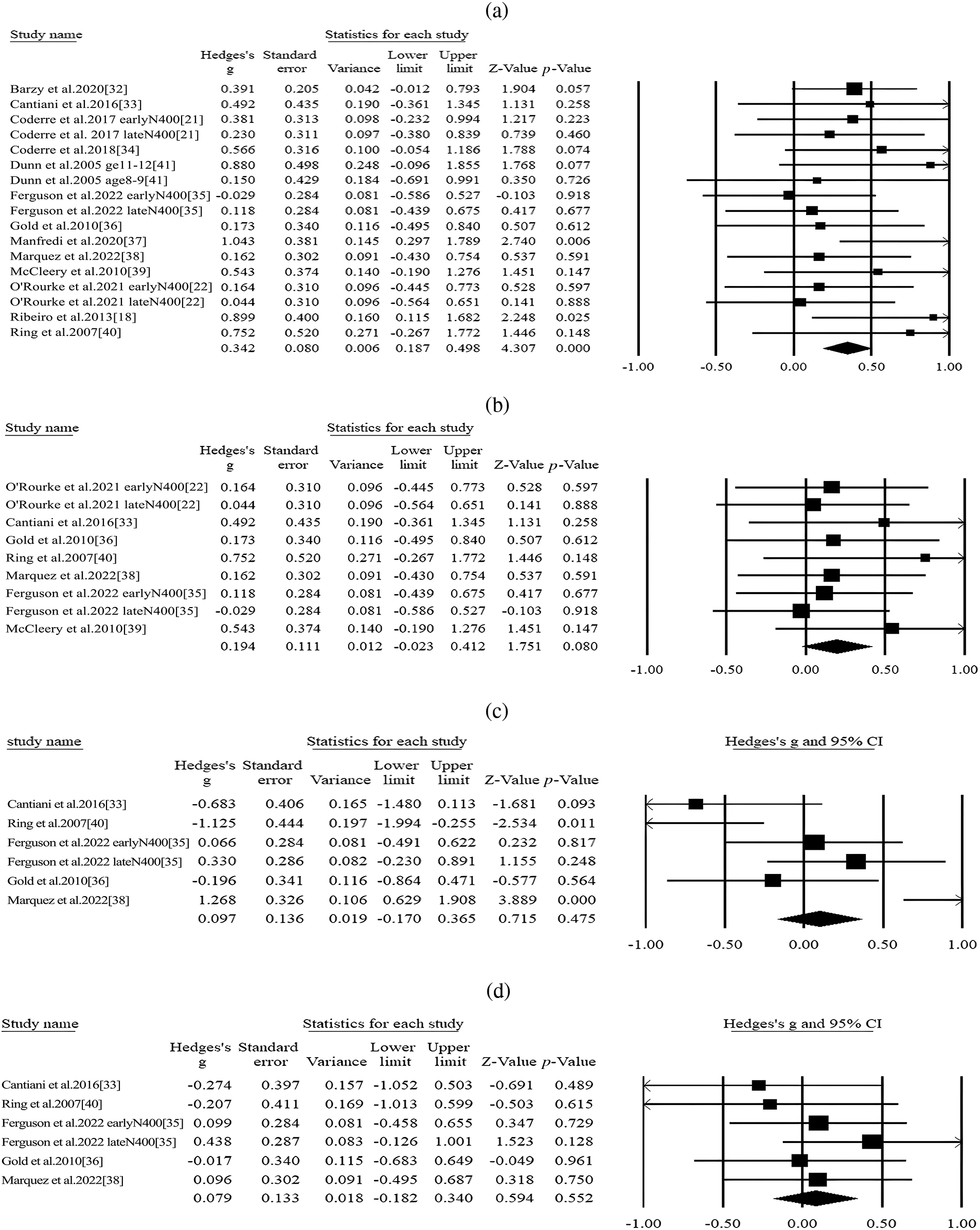
Figure 2: Forest plot of N400 amplitude for linguistic stimuli between ASD and controls.
The subgroup analysis was conducted as we assumed that the age, paradigm, diagnosis, level of verbal stimuli, modality, and electrode location might affect the N400 amplitude. The synthetic results of N400 amplitude were homogeneous in different ages, diagnoses, modalities, language levels, and paradigms. However, no significant effect size were found in the subgroups of Asperger syndrome, in-versus out-of-category words paradigm and Pz electrode site (Table 2), suggesting that N400 amplitude was comparable in the in-versus out of category words paradigm, Asperger’s syndrome and Pz electrode site subgroups between autistic and nonautistic people.
According to the meta-regression analysis, gender might be a potential moderator related to effect size (Z = 2.254, p = 0.024). The result suggested that with the increase of proportion of males in ASD group, the N400 amplitude showed a trend of decrease. However, age was not a potential moderator with nonsignificant effect size was found (Z = −1.358, p = 0.174). As the intellectual ability in autistic individuals, especially the language capacity, was highly heterogeneous, we considered “verbal IQ”, “non-verbal IQ”, “full-scale IQ”, and “receptive language” as potential moderators that might affect the effect size. The result of moderator analysis showed that verbal IQ accounted for significant variance in the N400 amplitude (Z = −2.024, p = 0.043), which indicated that verbal IQ could significantly affect the N400 amplitude. However, the influence of non-verbal IQ and receptive language ability on N400 amplitude was not significant (p < 0.05). In addition, we considered that the trials under each condition and autistic traits measured by AQ might be the potential moderators. The results of moderator analysis revealed that these moderators were not significantly related to effect size (p < 0.05, Table 3). The funnel is symmetrical, indicating that publication bias was not captured among the smaller and larger studies (Fig. 3). The p-value of the Egger test was 0.064, which confirmed that the results did not suffer from publication bias.
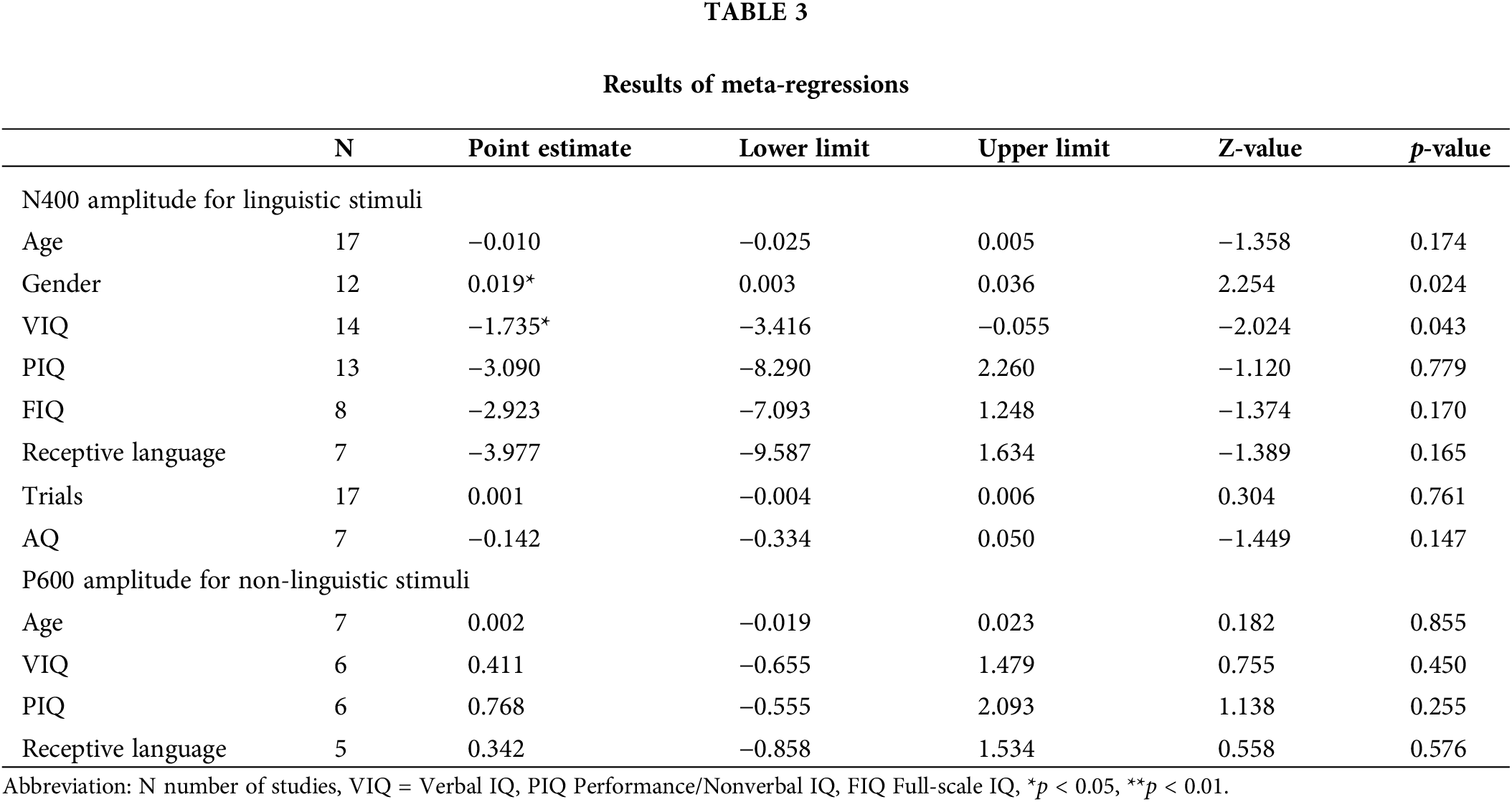
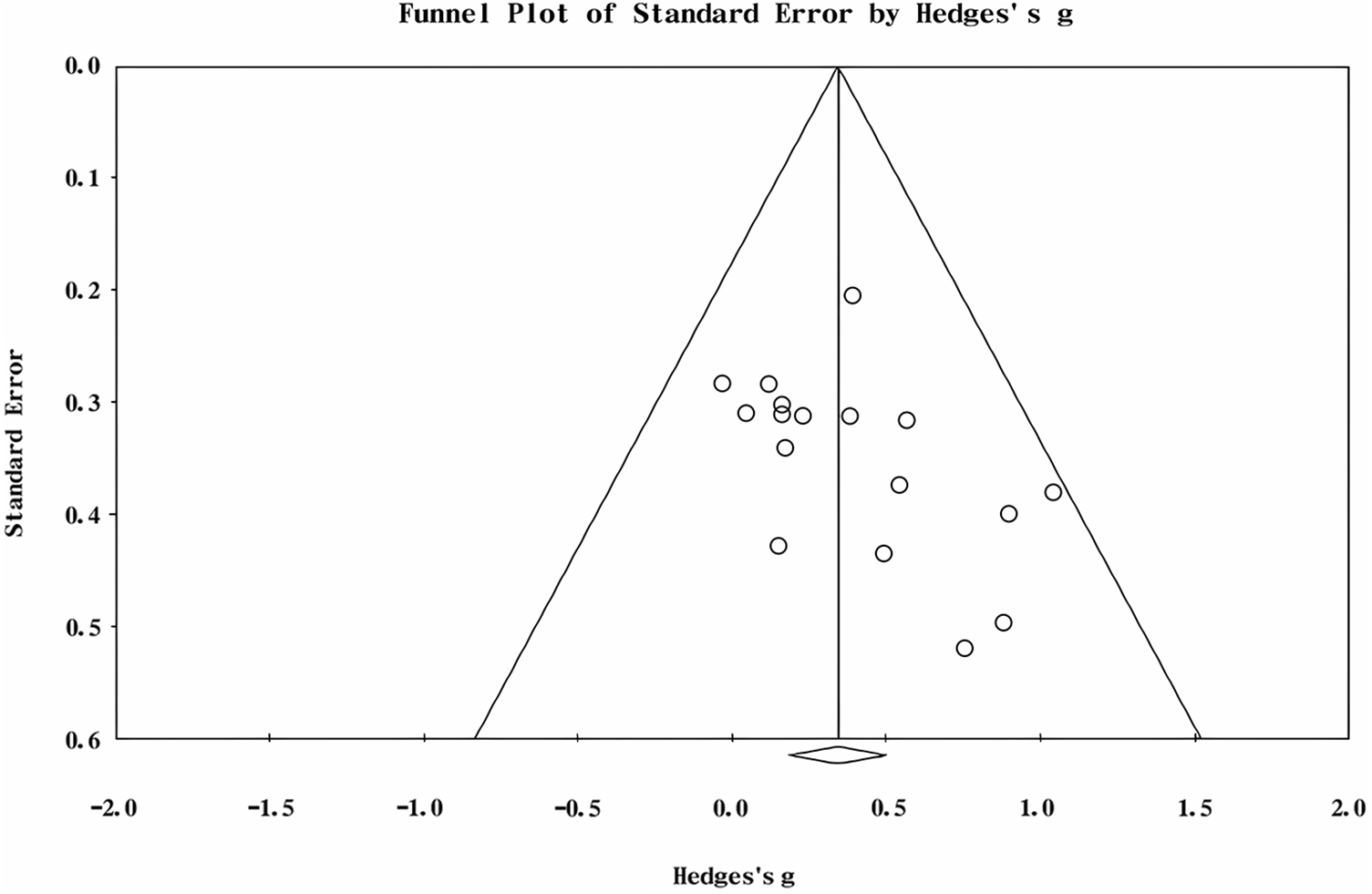
Figure 3: Funnel plot for publication bias of N400 amplitude for linguistic stimuli between ASD and controls.
N400 effect difference under linguistic stimulus
Among the articles, nine studies included a difference wave of N400 between incongruent and congruent conditions [22,33,35,36,38–40]. The fixed effect model was selected as insignificant heterogeneity (I2= 0.000%, Q = 1.751). After summarizing the outcome of N400 difference waves, the synthetic results revealed a marginally significant difference between the two groups (Hedges’ g = 0.194, 95% CI ranged from −0.23 to 0.412, p = 0.08) (Fig. 2b). The subgroup analysis of the electrode site and diagnosis revealed that neither of them would affect the heterogeneity, although the difference of N400 effect was more pronounced at the central electrode (Table 2).
Amplitudes of N400 in incongruent or congruent condition under linguistic stimulus
Five studies compared the N400 amplitude in congruent/related conditions between autistic and nonautistic groups [33,35,36,38,40]. The N400 amplitude under congruent condition of the two groups was comparable and the difference was statistically insignificant (Hedges’ g = −0.017, 95% CI ranged from −0.632 to 0.597, p = 0.955, I2 = 80.454%, Q = 25.581) (Fig. 2c). Similarly, five studies included the N400 amplitude under incongruent/unrelated conditions. The difference was found to be insignificant and the studies in the pool were homogenous (Hedges’ g = 0.079, 95% CI ranged from −0.182 to 0.340, p = 0.552, I2 = 0.000%, Q = 2.922) (Fig. 2d). The subgroup analysis of the electrode site indicated a more negative N400 amplitude in incongruent and congruent conditions at the parietal site, although the effect sizes were insignificant (Table 2).
P600 amplitude difference under linguistic stimulus
We included 7 articles and 8 studies in the meta-analysis that reported P600 amplitudes differences between autistic and nonautistic group [18,21,22,34,37,38,42]. According to the no statistical heterogeneity for the P600 amplitude under speech sound stimulation, the fixed effect model was applied for analysis (Q = 12.516, I2= 44.073). Among the 8 studies, only 2 studies [21,38] revealed a late positive potential and 6 studies reported a sustained negativity [18,22,34,37,42]. The difference of P600 amplitude between two groups was significant, indicating greater negativity was elicited in the nonautistic group compared to the autistic group (Hedges’ g = 0.289, 95% CI ranged from 0.059 to 0.518, p = 0.014, Fig. 4). As indicated by meta-regression, neither age nor intellectual ability showed a moderating effect (p > 0.05, Table 3).
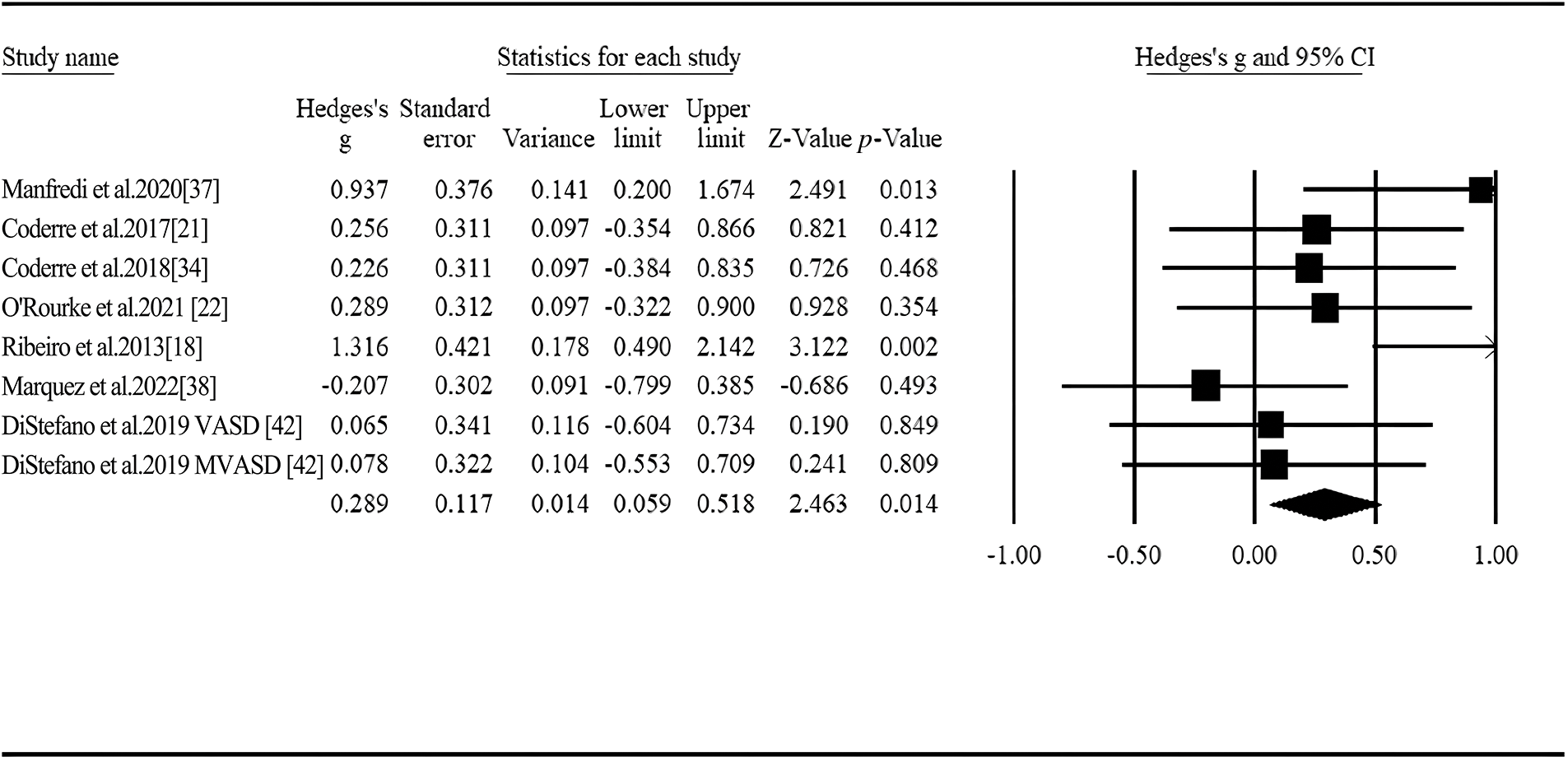
Figure 4: Forest plot of P600 amplitude for linguistic stimuli between ASD and controls.
ERP component elicited by non-linguistic stimulus
In a total of 5 article and 7 studies, non-linguistic stimuli such as music, pictures, and comics were applied to elicit N400 and P600 amplitudes [18,21,22,34,39]. Two effect sizes were computed to examine the response of non-linguistic stimulus: N400 amplitude and P600 amplitude.
N400 amplitude difference under non-linguistic stimulus
In a total of 5 articles and 7 studies, non-linguistic stimuli were applied to elicit N400 amplitude [18,21,22,34,39]. The available evidence was not enough to show a statistically significant difference between two groups in amplitudes of the N400 elicited by the non-linguistic stimuli (Hedges’ g = 0.025, 95% CI ranged from −0.217 to 0.268, p = 0.837, Fig. 5). The studies in the pool were homogenous (I2= 23.510%, Q = 7.844).
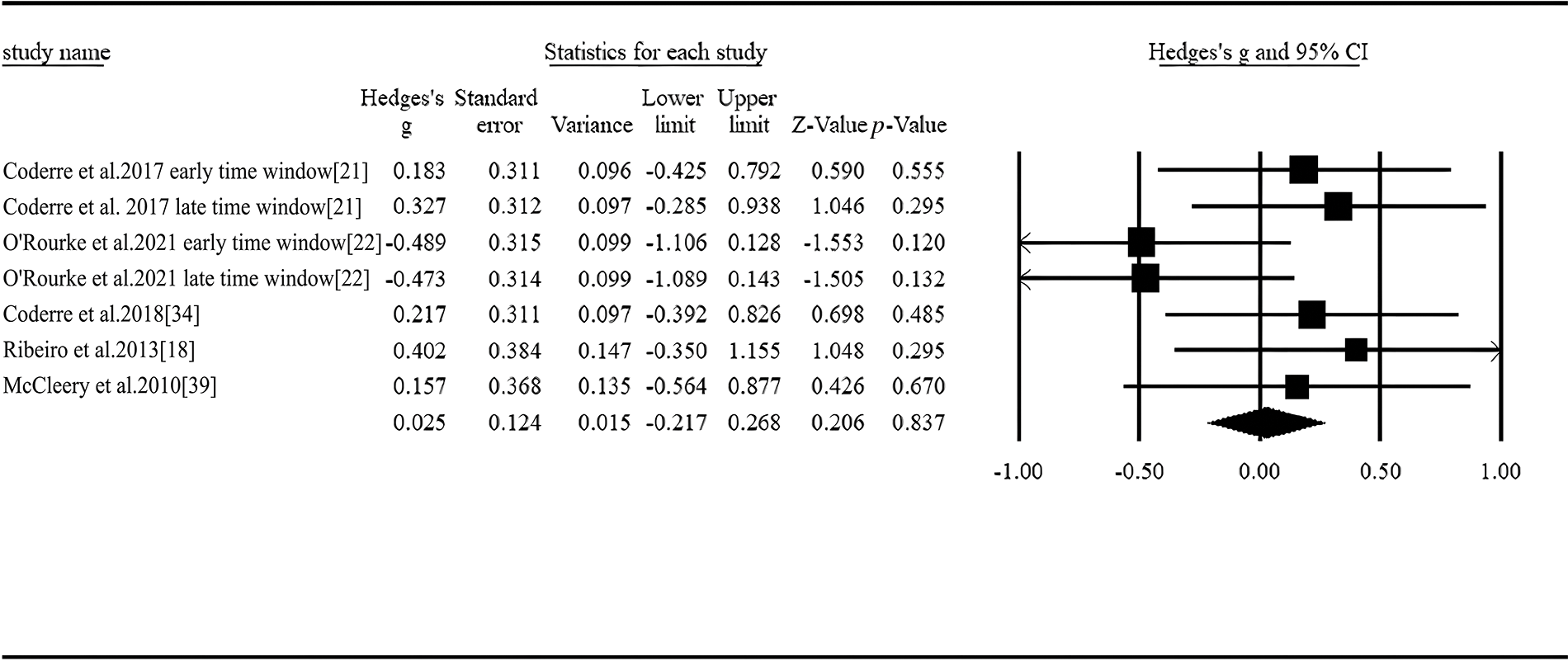
Figure 5: Forest plot of N400 amplitude for non-linguistic stimuli between ASD and controls.
P600 amplitude difference under non-linguistic stimulus
A total of 4 studies reported P600 amplitude difference under non-linguistic materials, the heterogeneity between studies was significant, and the random effect model was used for analysis (Q = 11.207, I2= 73.231) [18,21,22,34]. The pooled result showed that no significant difference between the autistic individuals and comparison group was found (Hedges’ g = −0.099, 95% CI ranged from −0.732 to 0.533, p = 0.271, Fig. 6).
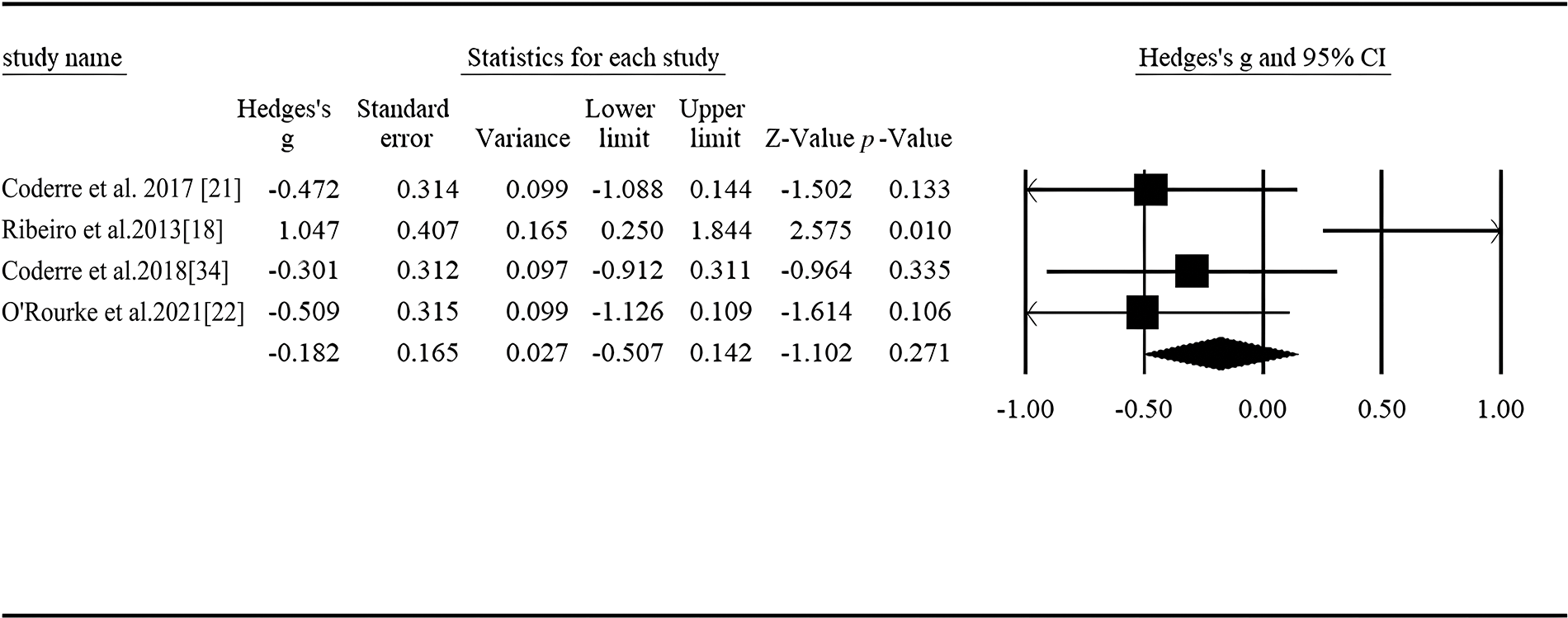
Figure 6: Forest plot of P600 amplitude for non-linguistic stimuli between ASD and controls.
This is the first meta-analysis examining semantic processing in autistic individuals from the ERPs perspective and providing a comprehensive overview of current knowledge about language-related ERPs in individuals of ASD. The meta-analysis pooled the results of 18 studies and showed that the autistic people tend to display a reduced overall N400 amplitude and N400 effect compared to nonautistic people. Besides, the reduced N400 amplitude is limited to linguistic materials and no statistically significant difference was observed under non-linguistic stimuli, indicating a language-specific deficit of semantic processing in autistic individuals.
N400 is a well-studied ERP component of semantic processing [10]. Our meta-analysis revealed an overall reduced N400 amplitude in the autistic group, which suggests an abnormal semantic processing in autistic individuals. According to the assumption of weak central coherence theory, autistic individuals only focus on fine-grained detail and have difficulty integrating information within the linguistic context [43]. Studies of functional magnetic resonance imaging (fMRI) and intracranial recordings have revealed that the posterior temporal cortex, anterior temporal cortex, and inferior frontal cortex play a role in the semantic processing of linguistic [9]. The evidence from fMRI studies also supported that there is inefficient activation of the brain areas associated with semantic processing in autistic individuals [44].
The N400 effect is the difference wave between congruent and incongruent conditions. Previous studies have shown that the N400 effect is sensitive to individual’s comprehensive skills [45]. In our meta-analysis, a reduced N400 effect was found in autistic individuals, which suggests a possible developmental disability of semantic processing in autistic individuals. Although no significant difference between two groups was found in incongruous or congruous conditions, autistic individuals showed a subtle reduced N400 amplitude in the incongruous conditions and enhanced negativity in the congruous condition. This trend could be the result of a reduced N400 effect in the autistic group. Attenuated N400 under incongruent condition indicates a decreased activation of the incongruent context in autistic individuals, and the enhanced N400 in congruent condition reflects the difficulty in using context to generate expectancies in autistic individuals [46].
In addition, there is a contentious debate on whether the impairment of semantic integration in autistic individuals is a language-specific or a global impairment. The synthesized result of our meta-analysis suggested a language-specific deficit, which can also be supported by the fMRI analysis indicating that individuals of ASD rely more on visual processing in the occipital cortex than verbal processing in the temporal cortex [44,47]. Some researchers speculated that pictorial semantic processing is superior to verbal semantic processing in ASD [23]. As the fundamental role of perception processing in language development, perception deficits are closely related to language ability. The ERP study of speech-specific perception deficits in ASD also supported the result of our meta-analysis [48].
Furthermore, the results of meta-regression revealed that the effect size of N400 amplitude might be affected by the verbal IQ, indicating the difference of N400 amplitude between two groups is negatively related to language capacity. A similar finding could also be observed in subgroup analysis. For people diagnosed with Asperger syndrome, a part of family of ASD that preserved cognitive and verbal facilities, the difference of N400 amplitude was comparable. The result is consistent with the literature reported previously that N400 had a solid relationship to vocabulary learning in children and sentence comprehension in adults [49]. Furthermore, gender may be another potential moderator affecting the N400 amplitude. The result may be explained by the fact that better linguistic abilities are more common in females than in males [50]. The group differences of N400 response were observed in semantic anomalies and semantic priming paradigms, which suggests the possible difficulty in integrating semantic context and facilitating lexical access in autistic individuals [9]. However, the inverted-out-of-category words task showed no significant difference between the two groups, which means that the lexical frequency effect is less sensitive in distinguishing autistic from the nonautistic group.
The P600 is a positive deflection of compositional semantic integration processes [51]. The P600 effect can be observed in responses to syntactic violations, semantic incongruency, and pragmatic anomalies. Some studies reported that P600 was not specifically related to the language process but was more likely to reflect a general monitoring process and modulated with instructions [52]. According to our meta-analysis from limited literature, contrary to Pijnacker et al. [53], the comparison group showed significantly greater negativity than autistic individuals under linguistic stimuli. One possible reason is that the effect of P600 overlapped by the sustained anterior negativity. Previous findings have reported that the sustained anterior negativity could be explained by the increased demands on working memory load and the decreased monitoring of violations. Therefore, the presence of negativity masked the expected P600 effect of plausibility [54]. In addition, language capacity did not affect the P600 component according to the result of meta-regression. These results indicated that P600 might not be a stable index of semantic processing in ASD, even though a significant difference was detected between the two groups. Further research is needed to explore the potential role of P600 in ASD. In the contrary, the attenuated amplitude of N400 might be a reliable component that related to the poor semantic ability in ASD. More work is needed to address whether N400 has the potential to be a marker of language impairment in ASD.
Our meta-analysis contains some limitations. First, only a few studies recruited autistic participants with minimally verbal ability [55]. The characteristic of N400 waveform in individuals of ASD with intellectual disability should be further explored. Second, the number of studies was relatively limited. Some researches were excluded from this study because no N400 response was elicited. Third, given that the amplitude response of N400 was most susceptible to manipulation and the latency was generally stable [10], only a limited number of studies reported the N400 latency. Therefore, our meta-analysis only incorporated the N400 amplitude index and did not include the latency indicators. Last but not least, as much of the literature reported previously, the ASD group shows atypical lateralization patterns of ERPs to speech stimuli [56]. The relationship between cerebral lateralization and language-related ERPs in autistic individuals should be considered in future studies.
This meta-analysis indicates an overall pattern of a reduced N400 amplitude and difference wave in autistic people under linguistic stimuli. The N400 amplitude is modulated by verbal intelligence and gender. In conclusion, our findings suggest a language-specific semantic processing deficiency in autistic people from some aspects of neurocognitive functions. The decrease of N400 amplitude might be a promising indication of poor language capacity in ASD.
Acknowledgement: Not applicate.
Funding Statement: The National Key Research and Development Program of China (Grant Number 2021ZD0202004).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and writing-original draft: Danfeng Yuan; data curation, writing-review and editing: Xiangyun Yang; validation, writing-review and editing: Lijuan Yang; conceptualization, funding acquisition, supervision, writing-review and editing: Zhanjiang Li. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analysed during this study are included in this published article, and its supplementary information files.
Ethics Approval: None.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th edition text revision (DSM-V) [Internet]. Washington: American Psychiatric Association; 2013. [Google Scholar]
2. Barbaro J, Dissanayake C. Developmental profiles of infants and toddlers with autism spectrum disorders identified prospectively in a community-based setting. J Autism Dev Disord [Internet]. 2012;42(9):1939–48. doi:https://doi.org/10.1007/s10803-012-1441-z. [Google Scholar] [PubMed] [CrossRef]
3. Hudry K, Leadbitter K, Temple K. Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. Int J Lang Commun Disord [Internet]. 2010;45(6):681–90. doi:https://doi.org/10.3109/13682820903461493. [Google Scholar] [PubMed] [CrossRef]
4. Wang S, Huang J. Semantic processing in language comprehension: evidence from multi-methodologies. Sheng li Xue Bao: [Acta physiologica Sinica] [Internet]. 2019;71(1):127–39. [Google Scholar] [PubMed]
5. Levinson S, Eisenhower A, Bush HH, Carter AS, Blacher J. Brief report: predicting social skills from semantic, syntactic, and pragmatic language among young children with autism spectrum disorder. J Autism Dev Disord [Internet]. 2020;50(11):4165–75. doi:https://doi.org/10.1007/s10803-020-04445-z. [Google Scholar] [PubMed] [CrossRef]
6. Molfese DL, Molfese VJ, Espy KA. The predictive use of event-related potentials in language development and the treatment of language disorders. Dev Neuropsychol [Internet]. 1999;16(3):373–7. doi:https://doi.org/10.1207/S15326942DN1603_19. [Google Scholar] [CrossRef]
7. Seyednozadi Z, Pishghadam R, Pishghadam M. Functional role of the N400 and P600 in language-related ERP studies with respect to semantic anomalies: an overview. Noro Psikiyatr Ars [Internet]. 2021;58(3):249–52. [Google Scholar] [PubMed]
8. Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science [Internet]. 1980;207(4427):203–5. doi:https://doi.org/10.1126/science.7350657. [Google Scholar] [PubMed] [CrossRef]
9. Lau EF, Phillips C, Poeppel DA. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci [Internet]. 2008;9(12):920–33. doi:https://doi.org/10.1038/nrn2532. [Google Scholar] [PubMed] [CrossRef]
10. Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annu Rev Psychol [Internet]. 2011;62(1):621–47. doi:https://doi.org/10.1146/annurev.psych.093008.131123. [Google Scholar] [PubMed] [CrossRef]
11. Quiroz GY. N400: an electrophysiological measure of semantic processing. Rev Neurol [Internet]. 2003;36(12):1176–80. [Google Scholar]
12. Schacht A, Sommer W, Shmuilovich O. Differential task effects on N400 and P600 elicited by semantic and syntactic violations. PLoS One [Internet]. 2014;9(3):e91226. doi:https://doi.org/10.1371/journal.pone.0091226. [Google Scholar] [PubMed] [CrossRef]
13. Geukes S, Huster RJ, Wollbrink A, Junghöfer M, Zwitserlood P, Dobel C. A large N400 but no BOLD effect—comparing source activations of semantic priming in simultaneous EEG-fMRI. PLoS One [Internet]. 2013;8(12):e84029. doi:https://doi.org/10.1371/journal.pone.0084029. [Google Scholar] [PubMed] [CrossRef]
14. Pastore RE, Crawley E, Skelly MA, Berens MS. Signal detection theory analyses of semantic priming in word recognition. J Exp Psychol Hum Percept Perform [Internet]. 2003;29(6):1251–66. doi:https://doi.org/10.1037/0096-1523.29.6.1251. [Google Scholar] [PubMed] [CrossRef]
15. van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Mem Cognit [Internet]. 1990;18(4):380–93. [Google Scholar] [PubMed]
16. Yum YN, Law SP. Interactions of age of acquisition and lexical frequency effects with phonological regularity: an ERP study. Psychophysiology [Internet]. 2019;56(10):e13433. doi:https://doi.org/10.1111/psyp.13433. [Google Scholar] [PubMed] [CrossRef]
17. Sitnikova T, Holcomb PJ, Kiyonaga KA. Two neurocognitive mechanisms of semantic integration during the comprehension of visual real-world events. J Cogn Neurosci [Internet]. 2008;20(11):2037–57. doi:https://doi.org/10.1162/jocn.2008.20143. [Google Scholar] [PubMed] [CrossRef]
18. Ribeiro TC, Valasek CA, Minati L, Boggio PS. Altered semantic integration in autism beyond language: a cross-modal event-related potentials study. Neuroreport [Internet]. 2013;24(8):414–8. doi:https://doi.org/10.1097/WNR.0b013e328361315e. [Google Scholar] [PubMed] [CrossRef]
19. Fishman I, Yam A, Bellugi U, Lincoln A, Mills D. Contrasting patterns of language-associated brain activity in autism and Williams syndrome. Soc Cogn Affect Neur [Internet]. 2011;6(5):630–8. doi:https://doi.org/10.1093/scan/nsq075. [Google Scholar] [PubMed] [CrossRef]
20. Méndez M, Sans O, Abril B, Valdizan JR. Event-related potentials (N 400) in autistic children. Clin Neurophysiol [Internet]. 2009;4(120):e136. [Google Scholar]
21. Coderre EL, Chernenok M, Gordon B, Ledoux K. Linguistic and non-linguistic semantic processing in individuals with autism spectrum disorders: an ERP study. J Autism Dev Disord [Internet]. 2017;47(3):795–812. doi:https://doi.org/10.1007/s10803-016-2985-0. [Google Scholar] [PubMed] [CrossRef]
22. O’Rourke E, Coderre EL. Implicit semantic processing of linguistic and non-linguistic stimuli in adults with autism spectrum disorder. J Autism Dev Disord [Internet]. 2021;51(8):2611–30. doi:https://doi.org/10.1007/s10803-020-04736-5. [Google Scholar] [PubMed] [CrossRef]
23. Kamio Y, Toichi M. Dual access to semantics in autism: is pictorial access superior to verbal access? J Child Psychol Psychiatry [Internet]. 2000;41(7):859–67. doi:https://doi.org/10.1111/1469-7610.00673. [Google Scholar] [CrossRef]
24. Sahyoun CP, Belliveau JW, Soulières I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia [Internet]. 2010;48(1):86–95. doi:https://doi.org/10.1016/j.neuropsychologia.2009.08.013. [Google Scholar] [PubMed] [CrossRef]
25. Desaunay P, Briant AR, Bowler DM. Memory in autism spectrum disorder: a meta-analysis of experimental studies. Psychol Bull [Internet]. 2020;146(5):377–410. doi:https://doi.org/10.1037/bul0000225. [Google Scholar] [PubMed] [CrossRef]
26. Leckey M, Federmeier KD. The P3b and P600(spositive contributions to language comprehension. Psychophysiology [Internet]. 2020;57(7):e13351. doi:https://doi.org/10.1111/psyp.13351. [Google Scholar] [PubMed] [CrossRef]
27. Shen W, Fiori-Duharcourt N, Isel F. Functional significance of the semantic P600: evidence from the event-related brain potential source localization. Neuroreport [Internet]. 2016;27(7):548–58. doi:https://doi.org/10.1097/WNR.0000000000000583. [Google Scholar] [PubMed] [CrossRef]
28. Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta-analyses that use standardized mean differences. Jama [Internet]. 2007;298(4):430–7. [Google Scholar]
29. Alexander RA, Scozzaro MJ, Borodkin LJ. Statistical and empirical examination of the chi-square test for homogeneity of correlations in meta-analysis. Psychol Bull [Internet]. 1989;106(2):329–31. doi:https://doi.org/10.1037/0033-2909.106.2.329. [Google Scholar] [CrossRef]
30. Sterne JA, Sutton AJ, Ioannidis JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ [Internet]. 2011;343:d4002. doi:https://doi.org/10.1136/bmj.d4002. [Google Scholar] [PubMed] [CrossRef]
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed) [Internet]. 1997;315(7109):629–34. doi:https://doi.org/10.1136/bmj.315.7109.629. [Google Scholar] [PubMed] [CrossRef]
32. Barzy M, Black J, Williams D, Ferguson HJ. Autistic adults anticipate and integrate meaning based on the speaker’s voice: evidence from eye-tracking and event-related potentials. J Exp Psychol Gen [Internet]. 2020;149(6):1097–115. doi:https://doi.org/10.1037/xge0000705. [Google Scholar] [PubMed] [CrossRef]
33. Cantiani C, Choudhury NA, Yu YH, Shafer VL, Schwartz RG, Benasich AA. From sensory perception to lexical-semantic processing: an ERP study in non-verbal children with autism. PLoS One [Internet]. 2016;11(8):e0161637. doi:https://doi.org/10.1371/journal.pone.0161637. [Google Scholar] [PubMed] [CrossRef]
34. Coderre EL, Cohn N, Slipher SK, Chernenok M, Ledoux K, Gordon B. Visual and linguistic narrative comprehension in autism spectrum disorders: neural evidence for modality-independent impairments. Brain Lang [Internet]. 2018;186(4):44–59. doi:https://doi.org/10.1016/j.bandl.2018.09.001. [Google Scholar] [PubMed] [CrossRef]
35. Ferguson HJ, Wimmer L, Black J, Barzy M, Williams D. Autistic adults are not impaired at maintaining or switching between counterfactual and factual worlds: an ERP study. J Autism Dev Disord [Internet]. 2022;52(1):349–360. doi:https://doi.org/10.1007/s10803-021-04939-4. [Google Scholar] [PubMed] [CrossRef]
36. Gold R, Faust M, Goldstein A. Semantic integration during metaphor comprehension in Asperger syndrome. Brain Lang [Internet]. 2010;113(3):124–34. doi:https://doi.org/10.1016/j.bandl.2010.03.002. [Google Scholar] [PubMed] [CrossRef]
37. Manfredi M, Cohn N, Sanchez Mello P, Fernandez E, Boggio PS. Visual and verbal narrative comprehension in children and adolescents with autism spectrum disorders: an ERP study. J Autism Dev Disord [Internet]. 2020;50(8):2658–72. doi:https://doi.org/10.1007/s10803-020-04374-x. [Google Scholar] [PubMed] [CrossRef]
38. Márquez-García AV, Vakorin VA, Kozhemiako N. Children with autism spectrum disorder show atypical electroence-phalographic response to processing contextual incongruencies. Sci Rep [Internet]. 2022;12(1):8948. doi:https://doi.org/10.1038/s41598-022-12475-z. [Google Scholar] [PubMed] [CrossRef]
39. McCleery JP, Ceponiene R, Burner KM, Townsend J, Kinnear M, Schreibman L. Neural correlates of verbal and nonverbal semantic integration in children with autism spectrum disorders. J Child Psychol Psychiatry [Internet]. 2010;51(3):277–86. doi:https://doi.org/10.1111/j.1469-7610.2009.02157.x. [Google Scholar] [PubMed] [CrossRef]
40. Ring H, Sharma S, Wheelwright S, Barrett G. An electro-physiological investigation of semantic incongruity processing by people with Asperger’s syndrome. J Autism Dev Disord [Internet]. 2007;37(2):281–90. doi:https://doi.org/10.1007/s10803-006-0167-1. [Google Scholar] [PubMed] [CrossRef]
41. Dunn MA, Bates JC. Developmental change in neutral processing of words by children with autism. J Autism Dev Disord [Internet]. 2005;35(3):361–76. doi:https://doi.org/10.1007/s10803-005-3304-3. [Google Scholar] [PubMed] [CrossRef]
42. DiStefano C, Senturk D, Jeste SS. ERP evidence of semantic processing in children with ASD. Dev Cogn Neurosci [Internet]. 2019;36(4):100640. doi:https://doi.org/10.1016/j.dcn.2019.100640. [Google Scholar] [PubMed] [CrossRef]
43. Eberhardt M, Nadig A. Reduced sensitivity to context in language comprehension: a characteristic of autism spectrum disorders or of poor structural language ability? Res Dev Disabil [Internet]. 2018;72:284–96. doi:https://doi.org/10.1016/j.ridd.2016.01.017. [Google Scholar] [PubMed] [CrossRef]
44. Phan L, Tariq A, Lam G, Pang EW, Alain C. The neurobiology of semantic processing in autism spectrum disorder: an activation likelihood estimation analysis. J Autism Dev Disord [Internet]. 2021;51(9):3266–79. doi:https://doi.org/10.1007/s10803-020-04794-9. [Google Scholar] [PubMed] [CrossRef]
45. Friedrich M, Friederici AD. Early N400 development and later language acquisition. Psychophysiology [Internet]. 2006;43(1):1–12. doi:https://doi.org/10.1111/j.1469-8986.2006.00381.x. [Google Scholar] [PubMed] [CrossRef]
46. Kumar N, Debruille JB. Semantics and N400: insights for schizophrenia. J Psychiatry Neurosci [Internet]. 2004;29(2):89–98. [Google Scholar] [PubMed]
47. Fan LY, Booth JR, Liu M, Chou TL, Gau SS. Developmental differences in neural connectivity for semantic processing in youths with autism. J Child Psychol Psychiatry [Internet]. 2021;62(9):1090–9. doi:https://doi.org/10.1111/jcpp.13373. [Google Scholar] [PubMed] [CrossRef]
48. Kuhl PK, Coffey-Corina S, Padden D, Munson J, Estes A, Dawson G. Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS One [Internet]. 2013;8(5):e64967. doi:https://doi.org/10.1371/journal.pone.0064967. [Google Scholar] [PubMed] [CrossRef]
49. Qi Z, Beach SD, Finn AS. Native-language N400 and P600 predict dissociable language-learning abilities in adults. Neuropsychologia [Internet]. 2016;98(1):177–91. doi:https://doi.org/10.1016/j.neuropsychologia.2016.10.005. [Google Scholar] [PubMed] [CrossRef]
50. Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry [Internet]. 2015;54(1):11–24. doi:https://doi.org/10.1016/j.jaac.2014.10.003 2015-01. [Google Scholar] [PubMed] [CrossRef]
51. Delogu F, Brouwer H, Crocker MW. Event-related potentials index lexical retrieval (N400) and integration (P600) during language comprehension. Brain Cogn [Internet]. 2019;135(1):103569. doi:https://doi.org/10.1016/j.bandc.2019.05.007. [Google Scholar] [PubMed] [CrossRef]
52. Regel S, Meyer L, Gunter TC. Distinguishing neurocognitive processes reflected by P600 effects: evidence from ERPs and neural oscillations. PLoS One [Internet]. 2014;9(5):e96840. doi:https://doi.org/10.1371/journal.pone.0096840. [Google Scholar] [PubMed] [CrossRef]
53. Pijnacker J, Geurts B, van Lambalgen M, Buitelaar J, Hagoort P. Exceptions and anomalies: an ERP study on context sensitivity in autism. Neuropsychologia [Internet]. 2010;48(10):2940–51. doi:https://doi.org/10.1016/j.neuropsychologia.2010.06.003. [Google Scholar] [PubMed] [CrossRef]
54. Delogu F, Brouwer H, Crocker MW. When components collide: spatiotemporal overlap of the N400 and P600 in language comprehension. Brain Res [Internet]. 2021;1766(9):147514. doi:https://doi.org/10.1016/j.brainres.2021.147514. [Google Scholar] [PubMed] [CrossRef]
55. Jolliffe T, Baron-Cohen S. Linguistic processing in high-functioning adults with autism or Asperger’s syndrome. is global coherence impaired? Psychol Med [Internet]. 2000;30(5):1169–87. doi:https://doi.org/10.1017/S003329179900241X. [Google Scholar] [PubMed] [CrossRef]
56. Finch KH, Seery AM, Talbott MR, Nelson CA. Tager-Flusberg H. Lateralization of ERPs to speech and handedness in the early development of Autism Spectrum Disorder. J Neurodev Disord [Internet]. 2017;9(1):4. doi:https://doi.org/10.1186/s11689-017-9185-x 2017. [Google Scholar] [PubMed] [CrossRef]
Supplementary Materials
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF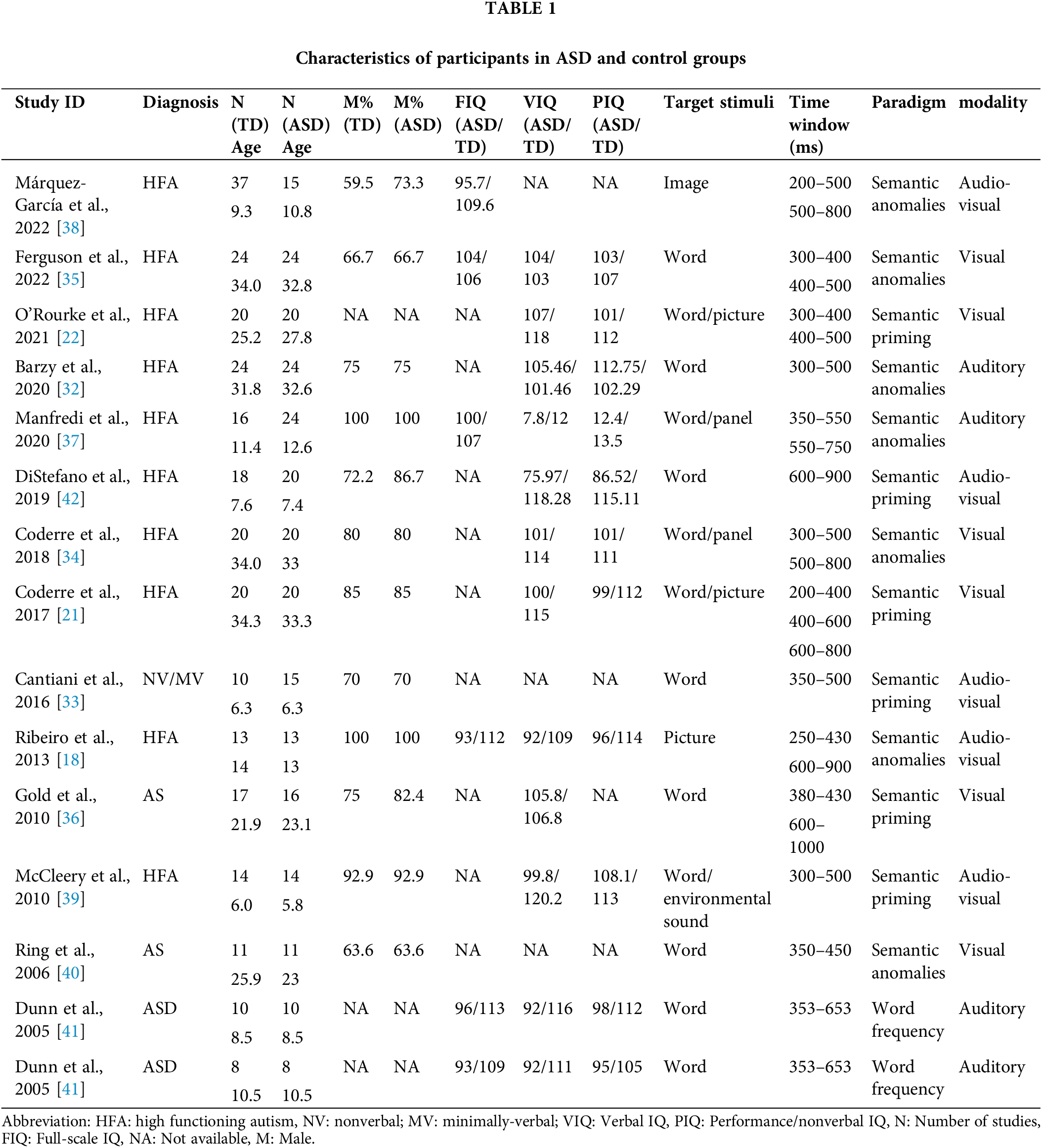
 Downloads
Downloads
 Citation Tools
Citation Tools