 Open Access
Open Access
REVIEW
Suicide in Digestive System Cancers: A Scoping Review
1
Department of Nursing, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan,
430000, China
2
School of Nursing, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430000, China
3
Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430000, China
* Corresponding Author: Deying Hu. Email:
International Journal of Mental Health Promotion 2023, 25(1), 1-20. https://doi.org/10.32604/ijmhp.2022.022578
Received 16 March 2022; Accepted 15 August 2022; Issue published 29 November 2022
Abstract
Digestive system cancers are important causes of morbidity and mortality worldwide. Cancer patients are more likely to commit suicide. The objective of this scoping review is to provide a comprehensive and updated summary of the existing literature on suicide among patients with digestive system cancers to identify the incidence and risk factors relevant to suicide in these populations. The PRISMA-Scr (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension protocol for scoping reviews) protocol was used. The review was based on relevant articles published prior to January 2022 in databases of Web of Science and PubMed. The authors identified 21 records that met the criteria for inclusion. Among the 21 articles, 18 (n = 85.7%) reported suicide risk factors, 21 (n = 100%) evaluated the incidence of suicide and 16 (n = 76.2%) involved the variation in suicide rates. Only one study comprehensively reported that the suicide rate for this population was 32.8 per 100,000 years and the standardized mortality ratio (SMR) was 1.91. Most suicides occurred in patients with pancreatic, esophageal, and gastric cancers. The factors associated with suicide in digestive system cancers included male gender, older age, the white race, single status, advanced stage of disease, and cancer metastasis. The most critical time for suicide was in the early post-diagnostic period. It is indispensable to identify suicide in these cancer patients, especially those with high-risk factors. In the future, more prospective research may be needed to provide more reliable support and care to prevent suicide.Keywords
It is well known that suicide is a serious global public health event. Suicide was a main cause of death in America, with an estimation of nearly 703,000 deaths due to suicide in 2019 [1,2]. The World Health Organization defines suicide as the act of an individual deliberately or voluntarily taking various means to end his or her life [3]. Beck et al. [4] divided suicide into three types: suicidal ideation, suicide attempt, and suicide death. Suicidal ideation is a desire to die but no action is taken. A suicide attempt is a deliberate act of self-destruction that does not result in death. Suicide death is a deliberate act of self-destruction that results in death. Diagnosis of cancer was a major stressor resulting in considerable adverse health outcomes [5,6]. Recently, a link between suicide and cancer has been noted. Previous evidence in systematic reviews has reflected an elevated suicide risk among cancer patients [7,8]. Misono et al. [9] reported that the suicide rate in cancer patients (31.4/100,000 person-years) was approximately 2-fold in comparison with the common population (16.7/100,000 person-years), particularly with certain cancers such as lung, stomach, mouth, throat, and larynx. Characteristics relevant to greater suicide rates included males, the white race, unmarried status, and advanced disease at diagnosis. Moreover, a recent study conducted by Zaorsky et al. [10] demonstrated an SMR of 4.44 in cancer patients. Digestive system tumors are common diseases that seriously threaten physical and mental health. The top 15 cancers in the world include colorectal, stomach, liver, esophageal, and pancreatic cancer [11]. Digestive system tumors have a high incidence and mortalities worldwide. For example, more than 1 million new stomach cancer cases and 769,000 deaths were estimated to occur in 2020. The incidence of colorectal cancer was 10.0%, and the mortality rate was 9.4%, ranking third and second, respectively. The incidence of liver cancer was 4.7%, and the death rate was 8.3%, ranking sixth and third, respectively [12]. Many patients suffering from digestive system cancers have poor prognoses and poor physical symptoms as well as a lower quality of life. One study indicated that this population had an increased suicide rate than the general U.S. population. Suicide rates in patients with esophageal cancer (SMR: 5.03) and pancreatic cancer (SMR: 5.28) were more than five times higher than those in ordinary people [13]. Some studies showed that digestive system cancer patients were at a high suicide risk in the early time after diagnosis, then it declined over time [5,10,14–16]. Risk factors of this group of people include sociodemographic features (e.g., gender, age, race, etc.) and clinical characteristics (e.g., period of diagnosis, stage, anatomic cancer sites, etc.). It is critical to identify patients at risk edge for suicide and carry out interventions to prevent suicidal behavior. However, as far as we know, there is no scoping review involving the risk factor analysis or suicide incidence among digestive system cancer patients. Therefore, we performed a scoping review to summarize the existing literature on risk factors and the incidence of suicide in patients suffering from digestive system cancers for better access to identification and supportive health care.
Scoping reviews are typically applied to (a) survey the extent, range, and nature of research; (b) determine the value of conducting a systematic review; (c) summarize and propagate research findings; (d) find potential gaps in the existing research [17]. We identified the purpose of this scoping review as 1) risk factors related to suicide in patients with digestive system cancers. 2) the incidence of suicide and its variations among these populations. This review was conducted in accordance with the PRISMA-ScR guidelines [18].
From November 2021 we searched online databases of Web of Science and PubMed without age restrictions to explore this research topic. We set a final retrieval date of December 31, 2021, for all database research available. We restricted the studies to human beings and the English language. The keywords and compositions were used to carry out each study. The retrieval strategy terminology was shown in the Table 1.

The criteria of included studies were as follows:
(1) data for patients who were diagnosed with digestive system cancers such as liver cancers, gastric cancers, colorectal cancers, pancreatic cancers or esophageal cancers;
(2) studies involved the risk factors associated with digestive system cancer such as age, gender, marital status, race, type of cancer, stage of cancer and so on;
(3) studies that reported the suicide incidence of digestive system cancers.
The following studies were excluded:
(1) studies that did not report any risk factor or incidence of digestive system cancer;
(2) studies without sufficient data on cancer patients;
(3) conference abstracts, comments, review, systematic review, meta-analysis and animal research;
(4) duplicate literature;
(5) articles not in English.
After searching the databases, we managed exported search results and removed duplicates by using EndNoteX9 (Clarivate Analytics, New York, NY, USA) software. Then, two investigators screened the titles and abstracts and evaluated the applicability of the criteria, respectively. The studies that met the minimum quality criteria were selected at this stage. Finally, they examined the selected studies for full text.
The “description-analysis” model proposed by Arksey et al. [17] was used to extract and summarize data. The lead author used a form in Microsoft Excel (Microsoft Corp) to extract the data consistently and accurately. All the included information was checked by the second author. The third author was responsible for resolving disagreements. We extracted the following information from the studies, including the name of the first author, the country of study object, year of publication, sample size, period of diagnosis, study design, source of data, main findings, and limitations. The extracted contents were shown in Table 2.
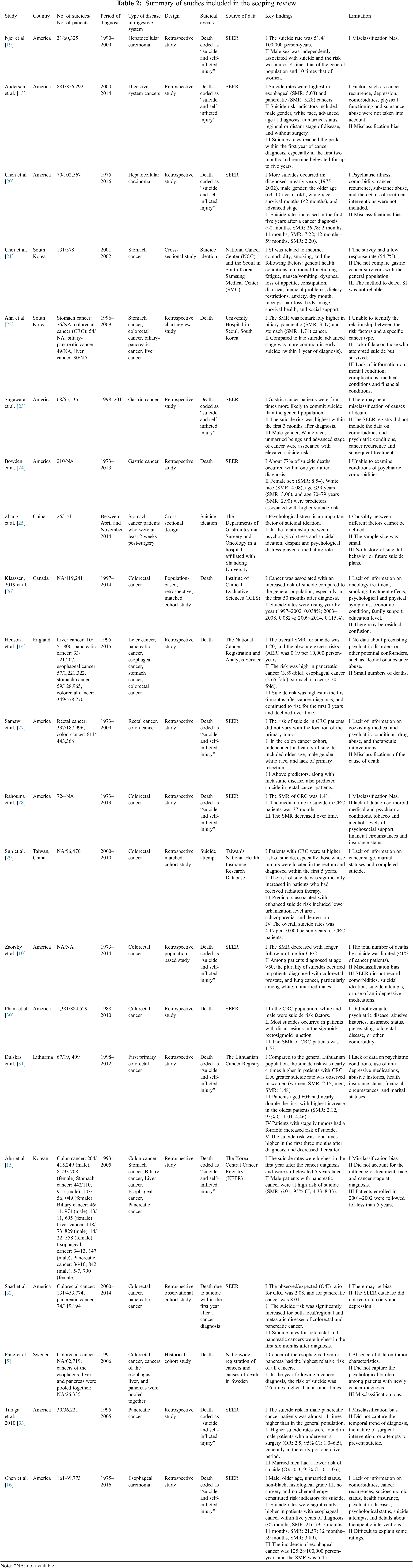
After searching the databases, we identified 2,472 records that documented suicide and digestive system cancer. 1,476 articles left after de-duplication. After title/abstract screening, 43 full-text articles were retained. Finally, we selected 21 studies that met the inclusion and exclusion criteria by reading the full texts. The flowchart in Fig. 1 illustrated the review process. These selected studies were published between 2010 and 2021. The total sample size of participants ranged from 32 to 1,221,322 diagnosed from 1973 to 2016. 12 studies were conducted in America, 3 in Korea, 2 in China, 1 in Canada, 1 in England, 1 in Lithuania, and 1 in Sweden. 19 were retrospectively studies (n = 90.5%) and 2 were cross-sectional studies.
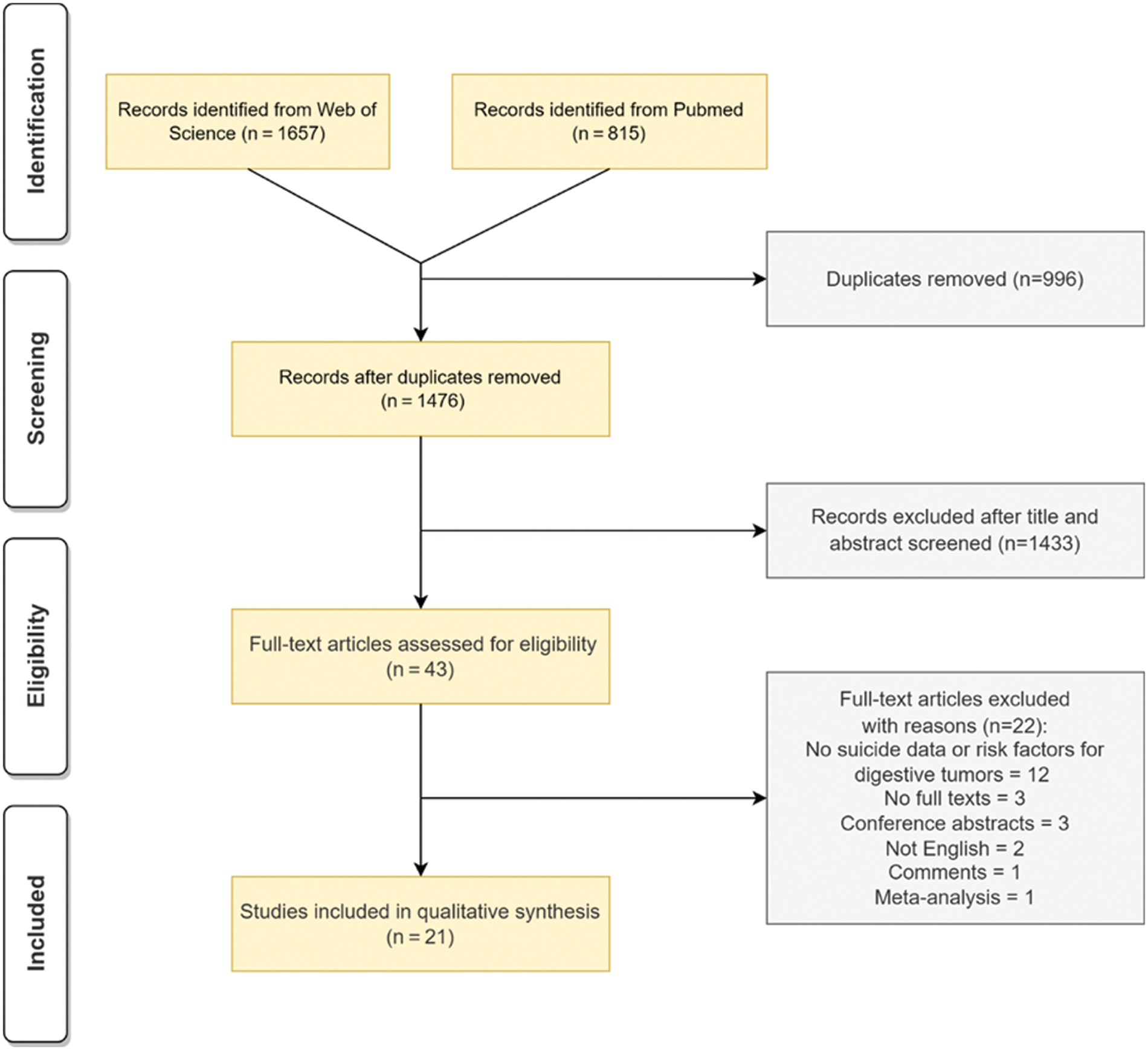
Figure 1: Flowchart of the review process
3.2 Prevalence of Suicide in Patients Suffering from Digestive System Cancers
Only a few studies have investigated suicide in the field of digestive tumors. Anderson et al. reviewed suicides among patients with digestive system cancers and identified the associated factors. Among 856,293 patients diagnosed with digestive tumors, a total of 881 were determined to have committed suicide. The suicide rate was 32.8 per 100,000 years, almost double that of ordinary people (SMR: 1.91). Suicide rates were highest in pancreatic (SMR: 5.28), esophageal (SMR: 5.03), and gastric (SMR: 2.84) cancer. Across all cancer sites, suicide risk was highest within the first year after the cancer diagnosis, especially in the first two months, but did not differ remarkably from the general population after five years [13]. A study on suicidal ideation in gastric cancer patients showed that 34.7% of 378 gastric cancer survivors had suicidal thoughts [21]. Sun et al. [29] reviewed the Taiwan National Health Insurance Research Database to explore suicide attempts among colorectal cancer patients. They found that compared to the control group, colorectal cancer patients had a significantly higher risk of suicide attempts. Additionally, the data suggested a signally elevated risk of suicide at follow-up <1 year and 1–5 years, but not ≥5 years.
3.3 Factors of Suicidal Behaviors
3.3.1 Factors of Suicidal Ideation and Attempts
Among the 21 articles, 2 articles revealed factors influencing suicidal ideation in patients with gastric cancer and 1 in colorectal cancer patients. Suicidal ideation in gastric cancer survivors was strongly associated with health-related quality of life such as diarrhea, hair loss, survival well-being and fatigue [21]. Zhang et al. [25] revealed that psychological strain was an important factor for suicidal ideation. In the relationship between psychological stress and suicidal ideation, feelings of hopelessness and psychological distress played mediating roles. In colorectal cancer patients, predictive factors related to a higher risk of suicidal attempts included lower urbanization level area, schizophrenia, depression and undergoing radiotherapy [29].
3.3.2 Factors of Suicidal Death
The interaction of many psychosocial, psychological, and neurobiological factors is thought to underlie suicidal behavior [34]. The risk of suicide in patients with digestive system cancers is strongly related to demographic and clinicopathological factors. The major risk factors were summarized in Table 3.
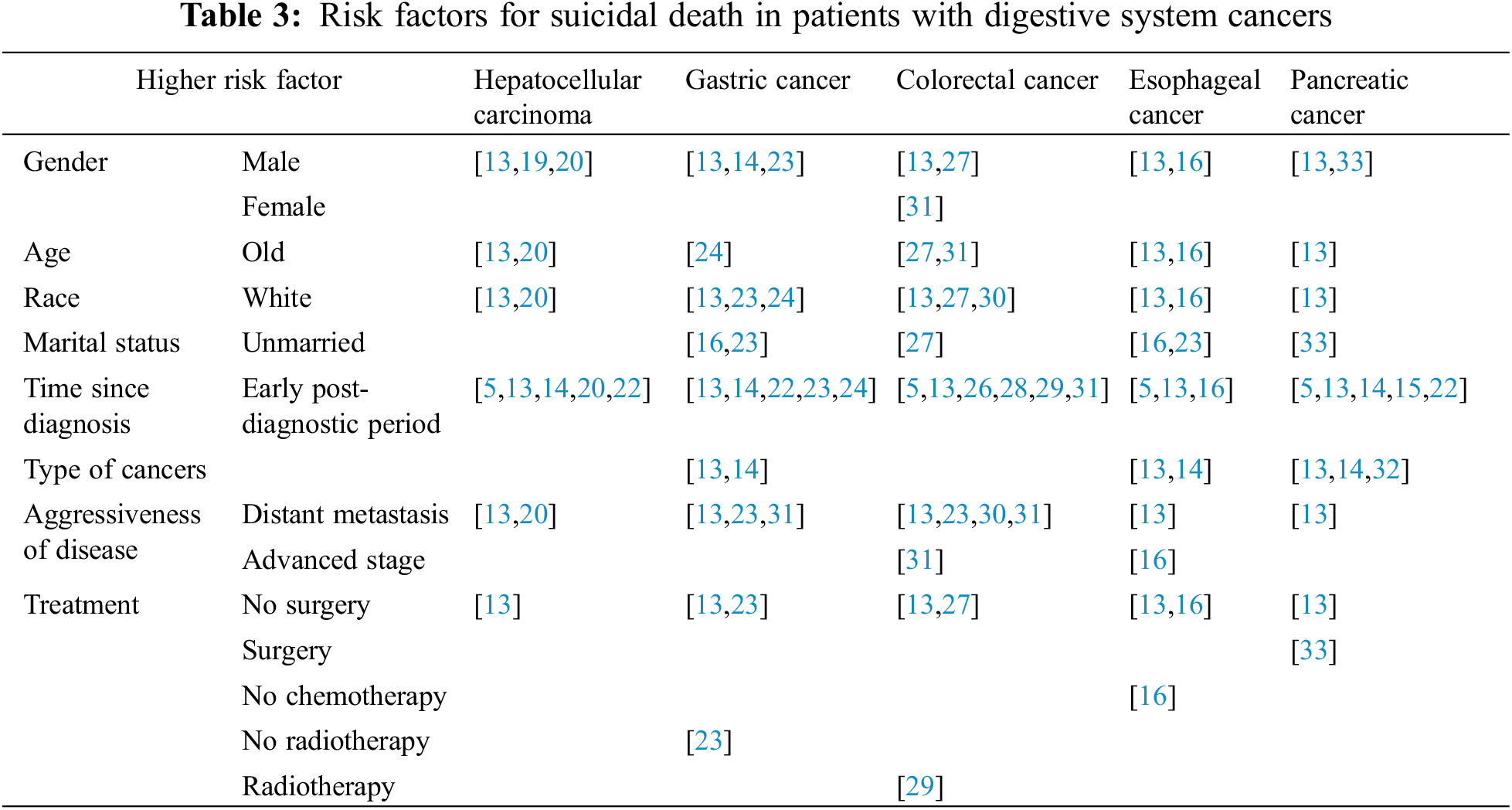
Gender and Suicide Risk in Digestive System Cancers
Gender is an important risk factor of suicide, especially the male gender [13]. Several studies revealed that the male gender was associated with a high risk of suicide in hepatocellular carcinoma patients [19,20]. This feature was also found in two articles on gastric cancer [14,23]. Likewise, male patients with pancreatic tumors had 11 times higher rates of suicide than the common population [33]. Besides that, in patients with esophageal cancer, men were more likely to have suicidal behavior than women, with a hazard ratio (HR) of 6.34 [16]. As in colorectal cancer, male gender was a strong predictor of suicide in both colon and rectal cancer cohorts. (Colon cancer HR: 6.82; rectal cancer HR: 9.91) [27]. However, a study conducted by Dulskas et al. reported that a higher suicide rate was observed in women (Women, SMR: 2.15; men, SMR: 1.48) [31].
Age
Anderson et al. [13] declared that SMRs rose with age at diagnosis and were highest in those aged 70+ years for liver, pancreatic and esophageal cancers. As for colon cancer, the authors found the highest SMR in patients younger than 50 and the lowest in patients aged 50–69. However, a study by Samawi et al. [27] reflected an opposite pattern. Advanced age (>70 years HR: 1.55) was a predictor of suicide in colorectal cancer patients. A similar result was observed in a Lithuania study, in which the suicide risk was almost twice higher among patients aged 60+ (SMR: 2.12) [31]. An investigation of hepatocellular tumors showed enhanced suicide risk in patients diagnosed with old age (63–105 vs. 0–55, HR: 2.28) [20]. Bowden et al. [24] discovered that age ≤39 years old (SMR: 3.06), and age between 70 to 79 years old (SMR: 2.90) were predictive factors associated with elevated incidence of suicide in gastric cancer patients. With regard to esophageal cancer, Chongfa Chen reviewed the SEER database and identified a rising trend of suicide in the elderly (70–105 vs. 0–55, HR: 2.69) [16].
Race
The white race was a predictor of suicide in digestive system tumors [13]. In a survey of patients with esophageal cancer from 1975–2016, the results demonstrated that the incidence of suicide in white was 135.25 per 100,000 person-years (vs. black race, HR: 6.64) [16]. The combined literature showed that the whites had significantly higher suicide rates and were more likely to commit suicide than other races [20,23,24,27,30].
Marital Status
Unmarried status was related to an increased risk of suicide in stomach cancer and esophageal cancer [16,23]. Among patients with pancreatic cancer, married men had a lower desire to commit suicide (OR: 0.3) [33]. Moreover, similar findings were observed in the colorectal cancer cohort, where single marital status (HR: 1.56; 95% CI: 1.14 to 2.13) might predict suicide [27].
Time Since Diagnosis
A diagnosis of cancer can increase the risk of suicide, especially within the first year after diagnosis [5]. SMRs remained elevated in patients with digestive system cancers between two months and five years after diagnosis, but were comparable to the general population after five years [13]. Ahn et al. [14,20,22] found that patients with liver cancer had a higher risk in the early post-diagnostic period. Similarly, this phenomenon was also observed in pancreatic cancer patients [14–15,22]. Five studies mentioned that stomach cancer patients tend to commit suicide in the early time after receiving a diagnosis [13–14,22–24]. About 77% of suicides occurred within the first year after diagnosis of gastric cancer [24]. Sun et al. [29] noted that colorectal cancer patients who were followed up for 1–5 years had a statistically higher risk of suicide than the non-colorectal cancer group. In the first 3 months after diagnosis of colorectal cancer, the suicide risk was four times higher than that of the general population [31]. A study based on SEER revealed a growing rate of suicide among esophageal cancer patients in the first five years following a cancer diagnosis (<2 months, SMR: 216.79; 2–11 months, SMR: 21.57; 12–59 months, SMR: 3.89) [16].
Type of Disease
Characteristics related to higher suicide incidence included the diagnosis of pancreatic (SMR: 5.28), esophageal (SMR: 5.03), or gastric cancer (SMR: 2.84) [13]. This was consistent with the study conducted by Henson et al. The authors discovered that these cancer types were associated with a particularly enhanced risk of suicide, with SMRs of 3.89 (pancreatic cancer), 2.65 (esophageal cancer) and 2.20 (stomach cancer) [14]. When assessing the predictors of suicide in colorectal cancer patients, Samawi et al. determined through univariate analysis that rectal-site cancer had a significantly elevated suicide risk [27,29]. In a study of 4,671,989 cancer patients, the results showed that the risk of suicide was particularly higher after a diagnosis of pancreatic or colorectal cancer. Pancreatic cancer had the highest increase in suicide rates after diagnosis, with the observed/expected (O/E) ratio of 8.01 [32].
Aggressiveness of Disease
Those with the distant-stage disease had higher suicidal tendencies and corresponding SMRs than those with localized or regional disease (Localized, SMR: 1.41; Regional, SMR: 1.76; Distant, SMR: 4.60) [13]. Prior review of the SEER database confirmed that the increasing stage of liver cancer was in connection with suicide (Localized, SMR: 2.14; Regional, SMR: 2.27; Distant, SMR: 4.00) [20]. Similar findings were found in studies of the stomach and colorectal cancer [23,31]. Sugawara et al. [23] calculated an SMR of 9.05 and an incidence rate ratio (IRR) of 2.90 for the distant stage of stomach cancer. Dulskas et al. pointed out that the distant spread of disease was one of the predictors of suicide. Colorectal cancer patients with stage IV had a fourfold increased suicide risk, whereas there was no significant elevation in localized tumors [31]. Interestingly, a survey based on the U.S. population illustrated that metastatic disease (M1) at diagnosis was associated with an enhanced risk of suicide in the rectal cancer group, but not in the colon cancer group [27]. Evidence suggested that rectal cancer patients with distal lesions were 52% more likely to suicide than those with proximal lesions (P < 0.0001) [30]. Regarding to esophageal cancer, patients with higher histological grades had rising suicidal tendencies (Grade III vs. Grade I, HR: 2.36) [16].
Influence of Treatment
Treatment regimens for tumors generally include surgery, chemotherapy and radiotherapy, etc. An article on suicide of digestive tumors reported greater suicide rates in those who did not have surgery [13]. Sugawara et al. found surgery and radiotherapy were protective factors for suicide in gastric cancer patients [23]. In an investigation based on esophageal cancer by Chen et al. [16], increasing suicide risk correlated with no surgery performed (no or unknown vs. yes, HR: 2.01) or no chemotherapy performed (no or unknown vs. yes, HR: 1.72) [16]. However, for pancreatic cancer, Turaga et al. [33] found that suicide tended to occur in patients who underwent operative interventions, generally in the first two months postoperatively. For colorectal cancer, a study from Taiwan revealed a growing trend of suicide in patients who received radiation therapy (adjusted hazard ratio, aHR: 1.48) [29]. Another study suggested that lack of primary tumor resection was related to higher suicide risk in colorectal cancer cohorts [27].
Cancer patients from different countries were at a growing risk of suicide [9,14,31,35,36]. Scholars in Italy (pooled SMR: 1.70) [35], Norway (HR: 2.50) [36], Lithuania (SMR: 1.62) [31], the U.K. (SMR: 1.20) [14], as well as the U.S. (SMR: 1.88) [9] had verified the higher suicide risk in cancer patients over the past few decades. This was consistent with the findings of studies included in our review that patients with digestive tumors had a higher suicide rate compared to the general population. The suicide rates in this population roughly doubled relative to the general population. Suicide incidence reached the peak within the first year after a cancer diagnosis, particularly in the first two months, and kept it high for five years, but dropped to the general level after five years [13]. Regarding suicidal ideation, a study from South Korea showed that 34.7% of 378 gastric cancer survivors had suicidal ideation [21]. In addition, there are no other reports of suicidal ideation in patients with digestive system tumors at present. The psychological problems of patients are unknown, and relevant studies are extremely lacking.
Suicidal ideation in stomach cancer survivors was strongly relevant to poorer health-related quality of life such as diarrhea, hair loss, survival well-being and fatigue [21]. Prior studies have confirmed a direct or indirect link between fatigue and suicide [37,38]. An early study indicated that diarrhea was positively correlated with suicidal thoughts. One convincing explanation was that diarrhea might cause unbearable physical discomfort and pain, thus restricting social activities and reducing the quality of life [39]. This state of isolation combined with physical distress can trigger depression, leading one to consider suicide. In addition, schizophrenia and depression were predictors of suicide attempts in colorectal cancer patients [29]. About 20% to 40% of cancer patients experience severe psychological distress, but less than 10% are identified and referred to specialist mental health services [40]. Cancer diagnosis and its treatment are known to cause depression. Psychiatric comorbidities have a strong influence on suicidal behavior induced by a cancer diagnosis. The relationship between psychiatric comorbidities and suicide in digestive system tumors remains to be further studied. There is still a lot of work to be done in the area of suicidal ideation in digestive tumors. A thorough and comprehensive analysis of suicidal ideation may help detect signs of suicidal behavior to avoid tragic events.
Many suicide risk factors in the general population can be extended to people with cancer, such as advanced age, male gender and other demographic characteristics [7–9,41]. Recent publications suggested that advanced age was a risk indicator of suicide for both the general population and cancer patients [5,42]. Some researchers reported a relatively high suicide risk in older cancer patients [9,43]. This conclusion was also observed in patients with digestive tumors, especially the liver, stomach, colorectal and esophageal cancers [16,20,24,27,31]. For the elderly, the combined negative effects of aging and cancer are much greater than those of other groups. The state of illness in elderly cancer patients may be more complex. Some patients are accompanied by other basic diseases. Their ability for self-care and physical activity are gradually reduced. The symptom burden in the elderly may be heavier than that in young people. At the same time, elderly cancer patients will also have varying degrees of depression, loneliness, and other psychological troubles, which may force them to commit suicide to get rid of the distress. Therefore, clinical workers should pay more attention to elderly cancer patients, observe their physiological and psychological changes, and carry out targeted suicide prevention. However, there were some exceptions. Other investigators found no significant correlation between the age of cancer patients and higher suicide risk [23,44,45]. Kroenke et al. [46,47] noted that younger breast cancer patients had a greater trend towards suicide than older patients. One possible explanation was younger persons reacted with more physical and mental deterioration, which exacerbated their suicide attempts.
In the global population, the suicide rate for men is twice as high as that for women. And suicide incidences among African men are three times that of South African women [2]. Studies have shown that men with serious physical illnesses, including other malignancies, are more likely to have suicidal ideation and attempts [48]. Most of the literature we included proved that the male gender was associated with a higher risk of suicide for digestive system cancers [13,14,16,19,20,23,27,33]. Although both men and women with cancer suffered similar pain and stress [49], alcohol consumption [50], smoking [51], and a sharp drop in family income might prompt men to think about suicide or even commit suicide [52]. Nevertheless, on the basis of data furnished by the Lithuanian Cancer Registry, Dulskas et al. [31] concluded that the female gender was a potential risk factor for suicide in colorectal cancer patients. Possible reasons might be significant changes in body image or changes in pelvic organ function after the stoma, which can seriously reduce the quality of life.
Many scholars have revealed that the most critical time for suicide is in the early post-diagnostic period [13,14,16,20,22–24,29,31]. Our scoping review about suicide in digestive tumors is consistent with prior investigations of other cancers in this aspect. The initial period after a cancer diagnosis was associated with a higher risk of suicide, with SMRs remaining elevated for up to five years, after which they usually decline [5,9,53,54]. For instance, Fang et al. [5] calculated that the relative risk of suicide was 12.6 during the first week. In the review by Llorente et al. [55] 80% of prostate cancer patients committed suicide within 6 months of diagnosis. Moreover, Lu et al. [56] pointed out that adolescents and young adults diagnosed with cancer had an immediate increased risk of suicidal behavior, especially in the first year after diagnosis (RR: 2.5, 95% CI 1.7–3.5). A cancer diagnosis can result in psychological stress, which may contribute to mental disorders such as depression, anxiety, and acute stress disorder. These stresses and psychotic disorders would have a direct or indirect impact on suicide mortality. Thus, it is necessary to carefully monitor and support cancer patients during these special periods.
Many researchers have defined the advanced stage as a predictor of suicide [5,6,9,49,54,57]. In a retrospective study by Ahn et al. [22], more than 70% of suicides in the first year occurred in cancer patients at stage 3 or 4. Vyssoki et al. [58] noted that cancer patients with locally advanced stage (SMR: 1.59) or metastasis (SMR: 4.07) had a higher suicide risk. The relationship between distant cancer stage and suicide was also observed in the digestive system [13,20,23,30,31]. Low histological grade indicated good differentiation of cancer cells, good prognosis, and improved living standard [59]. A study based on esophageal cancer showed a rising trend of suicide in patients with higher histological grades [16]. The desire for suicide in advanced cancer patients was likely multifactorial including physical condition, quality of life, and feelings of being a burden to others. Metastatic tumors often caused drastic pain, which made them suffer from severe psychological distress such as depression and anxiety. In addition, increased morbidity, decreased physical function, and the need for continuous treatment aggravated the symptoms of comorbid depression, and also put enormous stress on caregivers. As a result, these patients could turn to suicide as a strategy to get rid of psychological distress and relieve family pressure.
Discussions about the impact of different treatment options on suicide among cancer patients vary. Cancer patients who did not receive or refuse treatment were reported to have a higher suicide risk than patients who received specific treatment [60,61]. Anderson et al. [13] confirmed that digestive system cancer patients who received surgery were less likely to commit suicide than those who did not (SMR: 5.20, 95% CI: 4.64–5.81). Likewise, Samawi et al. [27] also considered no surgery as an independent risk factor. Some of the articles included in our review demonstrated that aggressive treatment was a protective factor for suicide in certain digestive tumors, such as gastric or esophageal tumors [16,23]. This may be attributed to more comfort and enhanced confidence in rehabilitation provided by the therapy, which alleviated the pain of cancer to some extent [62]. By contrast, in an investigation of pancreatic cancer, Turaga et al. [33] highlighted a higher propensity to suicide in patients who underwent operative intervention, generally in the first two months postoperatively. It has been proved that mortality may increase if the primary tumor is removed in the advanced stage of the disease [63,64]. Of course, more prospective studies on the effects of treatment are needed to clarify and provide better clinical recommendations.
Suicide represents a heavy health burden in digestive system cancer patients. Most of the studies selected were retrospective. Regretfully, there were no prospective studies in our review. The data or participants were mostly from developed countries, and we lacked information on cancer-related suicides in resource-limited countries. From the literature, we concluded that the suicide risk in patients suffering from digestive cancer was almost double that of ordinary people. Poor quality of life and psychological stress can contribute to suicidal ideation. Most suicides occurred in patients with esophageal, pancreatic and stomach cancers. Older age, male gender, white race, single status, advanced stage of disease, and cancer metastasis seem to constitute remarkable indicators of suicide in digestive system tumors. The suicide risk may last for long periods even after the treatment. I worth noting that the early periods following a cancer diagnosis are the critical time for suicide.
Beyond the above risk factors, suicide is a very complex behavior that can be influenced by the interplay of physiological, psychic, social and economic factors. Therefore, further research could take more factors into account, such as comorbidities, psychiatric conditions, substance abuse, cancer recurrences, economic status, education level, insurance status and so on. Despite the seriousness of the suicide, we found only a few studies directly focused on this issue in the population of digestive cancers, the majority of which were conducted in more recent years. In the future, larger prospective studies from more countries are needed to assess specific risk factors for suicide in patients with digestive tumors in different clinical cultural settings.
Various limitations of this scoping review need to be considered. One limitation is that several studies were conducted using the SEER database, so there may be an overlap between their results. Furthermore, since this is a scoping review, it only covers the scientific research achievements of the recent ten years and English articles published in mainstream journals in relevant fields. Some articles may have been missed when the search was undertaken.
In summary, identifying the suicide-related risk factors in digestive system cancer patients is an indispensable step in suicide prevention. It can not only save the lives of these patients, but also improve their quality of life. The conclusions of our review, coupled with further research and analysis, will be used to develop comprehensive screening strategies for early identification and interventions of cancer individuals at high suicide risk. Finally, we hope that healthcare providers will take more effective measures to support their clinical care and reduce suicide rates, which are matters of great urgency.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: D. H.; data collection: J. C., X. D.; analysis and interpretation of results: J. C., X. P.; draft manuscript preparation: J. C. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgement: We would like to thank School of Nursing, Tongji Medical College, Huazhong University of Science and Technology.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. McDowell, A. K., Lineberry, T. W., Bostwick, J. M. (2011). Practical suicide-risk management for the busy primary care physician. Mayo Clinic Proceedings, 86(8), 792–800. DOI 10.4065/mcp.2011.0076. [Google Scholar] [CrossRef]
2. World Health Organization (2021). Suicide worldwide in 2019: Global health estimates. [Google Scholar]
3. Turecki, G. B., David, A. (2016). Suicide and suicidal behaviour. The Lancet, 387(10024), 1227–1239. DOI 10.1016/S0140-6736(15)00234-2. [Google Scholar] [CrossRef]
4. Beck, A. T., Davis, J. H., Frederick, C. J. (1972). Classification and nomenclature. In: Suicide prevention in the seventies, Washington DC, USA: Government Printing Office. [Google Scholar]
5. Fang, F., Fall, K., Mittleman, M. A., Sparen, P., Ye, W. et al. (2012). Suicide and cardiovascular death after a cancer diagnosis. New England Journal of Medicine, 366(14), 1310–1318. DOI 10.1056/NEJMoa1110307. [Google Scholar] [CrossRef]
6. Nasseri, K., Mills, P. K., Mirshahidi, H. R., Moulton, L. H. (2012). Suicide in cancer patients in California, 1997–2006. Archives of Suicide Research, 16(4), 324–333. DOI 10.1080/13811118.2013.722056. [Google Scholar] [CrossRef]
7. Anguiano, L., Mayer, D. K., Piven, M. L., Rosenstein, D. (2012). A literature review of suicide in cancer patients. Cancer Nursing, 35(4), E14–26. DOI 10.1097/NCC.0b013e31822fc76c. [Google Scholar] [CrossRef]
8. Robson, A., Scrutton, F., Wilkinson, L., MacLeod, F. (2010). The risk of suicide in cancer patients: A review of the literature. Psychooncology, 19(12), 1250–1258. DOI 10.1002/pon.1717. [Google Scholar] [CrossRef]
9. Misono, S., Weiss, N. S., Fann, J. R., Redman, M., Yueh, B. (2008). Incidence of suicide in persons with cancer. Journal of Clinical Oncology, 26(29), 4731–4738. DOI 10.1200/JCO.2007.13.8941. [Google Scholar] [CrossRef]
10. Zaorsky, N. G., Zhang, Y., Tuanquin, L., Bluethmann, S. M., Park, H. S. et al. (2019). Suicide among cancer patients. Nature Communications, 10(1), 207. DOI 10.1038/s41467-018-08170-1. [Google Scholar] [CrossRef]
11. Islami, F., Ward, E. M., Sung, H., Cronin, K. A., Tangka, F. K. L. et al. (2021). Annual report to the nation on the status of cancer, part 1: National cancer statistics. Journal of the National Cancer Institute, 113(12), 1648–1669. DOI 10.1093/jnci/djab131. [Google Scholar] [CrossRef]
12. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I. et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. DOI 10.3322/caac.21660. [Google Scholar] [CrossRef]
13. Anderson, C., Park, E. M., Rosenstein, D. L., Nichols, H. B. (2018). Suicide rates among patients with cancers of the digestive system. Psychooncology, 27(9), 2274–2280. DOI 10.1002/pon.4827. [Google Scholar] [CrossRef]
14. Henson, K. E., Brock, R., Charnock, J., Wickramasinghe, B., Will, O. et al. (2019). Risk of suicide after cancer diagnosis in england. JAMA Psychiatry, 76(1), 51–60. DOI 10.1001/jamapsychiatry.2018.3181. [Google Scholar] [CrossRef]
15. Ahn, E., Shin, D. W., Cho, S. I., Park, S., Won, Y. J. et al. (2010). Suicide rates and risk factors among Korean cancer patients, 1993–2005. Cancer Epidemiol Biomarkers Prevention, 19(8), 2097–2105. DOI 10.1158/1055-9965.EPI-10-0261. [Google Scholar] [CrossRef]
16. Chen, C., Lin, H., Xu, F., Liu, J., Cai, Q. et al. (2021). Risk factors associated with suicide among esophageal carcinoma patients from 1975 to 2016. Scientific Reports, 11(1), 18766. DOI 10.1038/s41598-021-98260-w. [Google Scholar] [CrossRef]
17. Arksey, H., O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. DOI 10.1080/1364557032000119616. [Google Scholar] [CrossRef]
18. Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H. et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScRChecklist and explanation. Annals of Internal Medicine, 169(7), 467–473. DOI 10.7326/M18-0850. [Google Scholar] [CrossRef]
19. Njei, B. L., Joseph, K. (2013). Suicide among U.S. adults with hepatocellular carcinoma. Gastrointest Cancer Research, 6(1), 31–32. [Google Scholar]
20. Chen, C., Jiang, Y., Yang, F., Cai, Q., Liu, J. et al. (2021). Risk factors associated with suicide among hepatocellular carcinoma patients: A surveillance, epidemiology, and end results analysis. European Journal of Surgical Oncology, 47, 640–648. DOI 10.1016/j.ejso.2020.10.001. [Google Scholar] [CrossRef]
21. Choi, Y. N., Kim, Y. A., Yun, Y. H., Kim, S., Bae, J. M. et al. (2014). Suicide ideation in stomach cancer survivors and possible risk factors. Support Care Cancer, 22(2), 331–337. DOI 10.1007/s00520-013-1975-4. [Google Scholar] [CrossRef]
22. Ahn, M. H., Park, S., Lee, H. B., Ramsey, C. M., Na, R. et al. (2015). Suicide in cancer patients within the first year of diagnosis. Psychooncology, 24(5), 601–607. DOI 10.1002/pon.3705. [Google Scholar] [CrossRef]
23. Sugawara, A., Kunieda, E. (2016). Suicide in patients with gastric cancer: A population-based study. Japanese Journall of Clinical Oncology, 46(9), 850–855. DOI 10.1093/jjco/hyw075. [Google Scholar] [CrossRef]
24. Bowden, M. B., Walsh, N. J., Jones, A. J., Talukder, A. M., Lawson, A. G. et al. (2017). Demographic and clinical factors associated with suicide in gastric cancer in the United States. Journal of Gastrointestinal Oncology, 8(5), 897–901. DOI 10.21037/jgo.2017.08.02. [Google Scholar] [CrossRef]
25. Zhang, X., Zhang, J., Procter, N., Chen, X., Su, Y. et al. (2017). Suicidal ideation and psychological strain among patients diagnosed with stomach cancer: The mediation of psychopathological factors. Journal of Nervous and Mental Disease, 205(7), 550–557. DOI 10.1097/NMD.0000000000000679. [Google Scholar] [CrossRef]
26. Klaassen, Z., Wallis, C. J. D., Chandrasekar, T., Goldberg, H., Sayyid, R. K. et al. (2019). Cancer diagnosis and risk of suicide after accounting for prediagnosis psychiatric care: A matched-cohort study of patients with incident solid-organ malignancies. Cancer, 125(16), 2886–2895. DOI 10.1002/cncr.32146. [Google Scholar] [CrossRef]
27. Samawi, H. H., Shaheen, A. A., Tang, P. A., Heng, D. Y. C., Cheung, W. Y. et al. (2017). Risk and predictors of suicide in colorectal cancer patients: A surveillance. Epidemiology, and End Results Analysis. Current Oncology, 24(6), e513–e517. [Google Scholar]
28. Rahouma, M., Kamel, M., Abouarab, A., Eldessouki, I., Nasar, A. et al. (2018). Lung cancer patients have the highest malignancy-associated suicide rate in USA: A population-based analysis. Ecancermedicalscience, 12, 859. DOI 10.3332/ecancer.2018.859. [Google Scholar] [CrossRef]
29. Sun, L. M., Lin, C. L., Hsu, C. Y., Kao, C. H. (2018). Risk of suicide attempts among colorectal cancer patients: A nationwide population-based matched cohort study. Psychooncology, 27(12), 2794–2801. DOI 10.1002/pon.4891. [Google Scholar] [CrossRef]
30. Pham, T. T., Talukder, A. M., Walsh, N. J., Lawson, A. G., Jones, A. J. et al. (2019). Clinical and epidemiological factors associated with suicide in colorectal cancer. Support Care Cancer, 27(2), 617–621. DOI 10.1007/s00520-018-4354-3. [Google Scholar] [CrossRef]
31. Dulskas, A., Patasius, A., Kaceniene, A., Urbonas, V., Smailyte, G. (2019). Suicide risk among colorectal cancer patients in Lithuania. International Journal of Colorectal Disease, 34(3), 555–558. DOI 10.1007/s00384-018-03228-4. [Google Scholar] [CrossRef]
32. Saad, A. M., Gad, M. M., Al-Husseini, M. J., AlKhayat, M. A., Rachid, A. et al. (2019). Suicidal death within a year of a cancer diagnosis: A population-based study. Cancer, 125(6), 972–979. DOI 10.1002/cncr.31876. [Google Scholar] [CrossRef]
33. Turaga, K. K., Malafa, M. P., Jacobsen, P. B., Schell, M. J., Sarr, M. G. (2011). Suicide in patients with pancreatic cancer. Cancer, 117(3), 642–647. DOI 10.1002/cncr.25428. [Google Scholar] [CrossRef]
34. Joiner Jr, T. E., Brown, J. S., Wingate, L. R. (2005). The psychology and neurobiology of suicidal behavior. Annual Review of Psychology, 56, 287–314. DOI 10.1146/annurev.psych.56.091103.070320. [Google Scholar] [CrossRef]
35. Ravaioli, A., Crocetti, E., Mancini, S., Baldacchini, F., Giuliani, O. et al. (2020). Suicide death among cancer patients: New data from northern Italy, systematic review of the last 22 years and meta-analysis. European Journal of Cancer, 125, 104–113. DOI 10.1016/j.ejca.2019.08.019. [Google Scholar] [CrossRef]
36. Gunnes, M. W., Lie, R. T., Bjorge, T., Ghaderi, S., Syse, A. et al. (2017). Suicide and violent deaths in survivors of cancer in childhood, adolescence and young adulthood–A national cohort study. International Journal of Cancer, 140(3), 575–580. DOI 10.1002/ijc.30474. [Google Scholar] [CrossRef]
37. Nakao, M., Yamanaka, G., Kuboki, T. (2002). Suicidal ideation and somatic symptoms of patients with mind/body distress in a Japanese psychosomatic clinic. Suicide and Life-Threatening Behavior, 32(1), 80–90. DOI 10.1521/suli.32.1.80.22179. [Google Scholar] [CrossRef]
38. Vilhjalmsson, R., Kristjansdottir, G., Sveinbjarnardottir, E. (1998). Factors associated with suicide ideation in adults. Social Psychiatry and Psychiatric Epidemiology, 33(3), 97–103. DOI 10.1007/s001270050028. [Google Scholar] [CrossRef]
39. Yoshimasu, K., Fukumoto, J., Takemura, S., Shiozaki, M., Yamamoto, H. et al. (2011). Subjective symptoms related to suicide risk in Japanese male police officers. Suicidology Online, 2, 38–47. [Google Scholar]
40. Holland, J. C., Alici, Y. (2010). Management of distress in cancer patients. Journal of Supportive Oncology, 8(1), 4–12. [Google Scholar]
41. Siracuse, B. L., Gorgy, G., Ruskin, J., Beebe, K. S. (2017). What is the incidence of suicide in patients with bone and soft tissue cancer?: Suicide and sarcoma. Clinical Orthopaedics and Related Research, 475(5), 1439–1445. DOI 10.1007/s11999-016-5171-y. [Google Scholar] [CrossRef]
42. Brower, V. (2016). Suicide in survivors of childhood and young adult cancers. The Lancet Oncology, 17(12), E522–E522. DOI 10.1016/S1470-2045(16)30554-X. [Google Scholar] [CrossRef]
43. Smailyte, G., Jasilionis, D., Kaceniene, A., Krilaviciute, A., Ambrozaitiene, D. et al. (2013). Suicides among cancer patients in Lithuania: A population-based census-linked study. Cancer Epidemiology, 37(5), 714–718. DOI 10.1016/j.canep.2013.05.009. [Google Scholar] [CrossRef]
44. Yousaf, U., Christensen, M. L., Engholm, G., Storm, H. H. (2005). Suicides among Danish cancer patients 1971–1999. British Journal of Cancer, 92(6), 995–1000. DOI 10.1038/sj.bjc.6602424. [Google Scholar] [CrossRef]
45. Johnson, T. V., Garlow, S. J., Brawley, O. W., Master, V. A. (2012). Peak window of suicides occurs within the first month of diagnosis: Implications for clinical oncology. Psychooncology, 21(4), 351–356. DOI 10.1002/pon.1905. [Google Scholar] [CrossRef]
46. Kroenke, C. H., Rosner, B., Chen, W. Y., Kawachi, I., Colditz, G. A. et al. (2004). Functional impact of breast cancer by age at diagnosis. Journal of Clinical Oncology, 22(10), 1849–1856. DOI 10.1200/JCO.2004.04.173. [Google Scholar] [CrossRef]
47. Gaitanidis, A., Alevizakos, M., Pitiakoudis, M., Wiggins, D. (2018). Trends in incidence and associated risk factors of suicide mortality among breast cancer patients. Psychooncology, 27(5), 1450–1456. DOI 10.1002/pon.4570. [Google Scholar] [CrossRef]
48. Waern, M., Rubenowitz, E., Runeson, B., Skoog, I., Wilhelmson, K. et al. (2002). Burden of illness and suicide in elderly people: Case-control study. BMJ, 324(7350), 1355. DOI 10.1136/bmj.324.7350.1355. [Google Scholar] [CrossRef]
49. Kendal, W. S. (2007). Suicide and cancer: A gender-comparative study. Annals of Oncology, 18(2), 381–387. DOI 10.1093/annonc/mdl385. [Google Scholar] [CrossRef]
50. Zaridze, D., Lewington, S., Boroda, A., Scélo, G., Karpov, R. et al. (2014). Alcohol and mortality in Russia: Prospective observational study of 151 000 adults. The Lancet, 383(9927), 1465–1473. DOI 10.1016/S0140-6736(13)62247-3. [Google Scholar] [CrossRef]
51. Kessler, R. C., Borges, G., Sampson, N., Miller, M., Nock, M. K. (2009). The association between smoking and subsequent suicide-related outcomes in the national comorbidity survey panel sample. Molecular Psychiatry, 14(12), 1132–1142. DOI 10.1038/mp.2008.78. [Google Scholar] [CrossRef]
52. Iemmi, V., Bantjes, J., Coast, E., Channer, K., Leone, T. et al. (2016). Suicide and poverty in low-income and middle-income countries: A systematic review. The Lancet Psychiatry, 3(8), 774–783. DOI 10.1016/S2215-0366(16)30066-9. [Google Scholar] [CrossRef]
53. Allebeck, P. (1989). Increased suicide rate in cancer patients A cohort study based on the Swedish cancer-environment register. Journal of Clinical Epidemiology, 42(7), 611–616. DOI 10.1016/0895-4356(89)90003-6. [Google Scholar] [CrossRef]
54. Hem, E., Loge, J. H., Haldorsen, T., Ekeberg, O. (2004). Suicide risk in cancer patients from 1960 to 1999. Journal of Clinical Oncology, 22(20), 4209–4216. DOI 10.1200/JCO.2004.02.052. [Google Scholar] [CrossRef]
55. Llorente, M. D., Burke, M., Gregory, G. R., Bosworth, H. B., Grambow, S. C. et al. (2005). Prostate cancer: A significant risk factor for late-life suicide. American Journal of Geriatric Psychiatry, 13(3), 195–201. DOI 10.1097/00019442-200503000-00004. [Google Scholar] [CrossRef]
56. Lu, D., Fall, K., Sparen, P., Ye, W., Adami, H. O. et al. (2013). Suicide and suicide attempt after a cancer diagnosis among young individuals. Annals of Oncology, 24(12), 3112–3117. DOI 10.1093/annonc/mdt415. [Google Scholar] [CrossRef]
57. Miller, M., Mogun, H., Azrael, D., Hempstead, K., Solomon, D. H. (2008). Cancer and the risk of suicide in older Americans. Journal of Clinical Oncology, 26(29), 4720–4724. DOI 10.1200/JCO.2007.14.3990. [Google Scholar] [CrossRef]
58. Vyssoki, B., Gleiss, A., Rockett, I. R., Hackl, M., Leitner, B. et al. (2015). Suicide among 915,303 Austrian cancer patients: Who is at risk? Journal of Affective Disorders, 175, 287–291. DOI 10.1016/j.jad.2015.01.028. [Google Scholar] [CrossRef]
59. Simpson, W. G., Klaassen, Z., Jen, R. P., Hughes, W. M., Neal, D. E. et al. (2018). Analysis of suicide risk in patients with penile cancer and review of the literature. Clinical Genitourinary Cancer, 16(2), e257–e261. DOI 10.1016/j.clgc.2017.09.011. [Google Scholar] [CrossRef]
60. Urban, D., Rao, A., Bressel, M., Neiger, D., Solomon, B. et al. (2013). Suicide in lung cancer: Who is at risk? Chest, 144(4), 1245–1252. DOI 10.1378/chest.12-2986. [Google Scholar] [CrossRef]
61. Carlsson, S., Sandin, F., Fall, K., Lambe, M., Adolfsson, J. et al. (2013). Risk of suicide in men with low-risk prostate cancer. European Journal of Cancer, 49(7), 1588–1599. DOI 10.1016/j.ejca.2012.12.018. [Google Scholar] [CrossRef]
62. Zhang, X., Sun, S., Peng, P., Ma, F., Tang, F. (2021). Prediction of risk of suicide death among lung cancer patients after the cancer diagnosis. Journal of Affective Disorders, 292, 448–453. DOI 10.1016/j.jad.2021.05.123. [Google Scholar] [CrossRef]
63. Clancy, C., Burke, J. P., Barry, M., Kalady, M. F., Calvin Coffey, J. (2014). A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Annals of Surgical Oncology, 21(12), 3900–3908. DOI 10.1245/s10434-014-3805-4. [Google Scholar] [CrossRef]
64. Ahmed, S., Leis, A., Fields, A., Chandra-Kanthan, S., Haider, K. et al. (2014). Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer, 120(5), 683–691. DOI 10.1002/cncr.28464. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools