

 | International Journal of Mental Health Promotion |  |
DOI: 10.32604/ijmhp.2022.021361
ARTICLE
The Effects of Therapeutic Horseback Riding Program on Motor Skills in Children with Autism Spectrum Disorder
1School of Physical Education, Shenzhen University, Shenzhen, 518060, China
2Department of Sports Science, Hefei Normal University, Hefei, 230601, China
3Body-Brain-Mind Laboratory, School of Psychology, Shenzhen University, Shenzhen, 518060, China
4School of Nursing, Psychotherapy and Community Health, Dublin City University, Glasnevin, Dublin 9, Ireland
5School of Rehabilitation, Sport and Psychology, AECC University College, Bournemouth, BH5S2DF, UK
6School of Sport Social Science, Shandong Sport University, Jinan, 250102, China
7Key Laboratory of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, East China Normal University, Shanghai, 200241, China
*Corresponding Author: Liye Zou. Email: liyezou123@gmail.com
Received: 10 January 2022; Accepted: 28 February 2022
Abstract: Therapeutic horseback riding (THR) as an animal-assisted intervention is one of the innovative approaches emerging in the treatment for children with autism spectrum disorder (ASD). The current study was designed to investigate the effects of a 12-week, twice a week THR program on motor skills in sixty-eight children with ASD aged 5–10 years old. All participants selected met the DSM-V criteria for ASD, and a total of fifty-three participants completed the study. A randomized controlled trial design was utilized for the study. Data was collected via a pre-THR test, interim-THR test, and post-THR test to investigate the possible changes in motor skills throughout the 12-week THR program. Results showed that the THR program significantly improved overall motor skills across time points (p < 0.05) and sub-skills of run, gallop and two-hand catch (as compared to the control group, p < 0.05). In conclusion, the THR program may be an effective option for improving motor skills in children with ASD and further investigation with a longer period of intervention is warranted.
Keywords: Therapeutic horseback riding; animal-assisted intervention; motor skills; children with ASD; autistic; developmental disability; equine-assisted activities and therapies
Autism spectrum disorder (ASD) is an increasingly common neurodevelopmental disorder characterized by social communication difficulties and restricted, and repetitive patterns of behaviors, interests, and activities [1]. The incidence of ASD has been growing dramatically over the past couple of decades, with estimates of 1 in 44 children diagnosed with ASD aged 8 years old in the United States in 2018, and is more common in boys [2]. The current trend in China is also a rapid increase in the prevalence of children diagnosed with ASD, in line with the global diagnostic trends. A wide range of evidence-based treatments have been developed for children with ASD, primarily targeting the core deficits related to social communication and certain typical behaviors [3]. However, there are limited interventions targeting motor skills as the primary outcome for children with ASD [4].
Accumulating evidence indicates that up to 83% of children with ASD have varying degrees of poor motor skills and delayed motor development as compared with typically developing children [5–12]. Such motor dysfunction or deficits have been observed on measures of gross and fine motor skill, coordination, balance, gait pattern, postural control, joint flexibility and movement speed [13–15]. Motor skill deficits can reduce the opportunities for participation in physical activity, increasing the rate of sedentary-related diseases [16] and possibly contributes to social isolation, anxiety, and emotional challenges for children with ASD and their families [17]. Therefore, it is important to monitor motor competence in children with ASD and address motor skill deficits through targeted interventions.
Equine-assisted activities and therapies (EAAT) are innovative, complementary, and alternative approaches for symptomatic management of children with ASD [3,18]. Therapeutic horseback riding (THR) is a type of EAAT that has a wider range of targeted therapeutic purposes than hippotherapy, and it takes advantage of equine rhythmic movement which could support improvement in the participants’ various functional goals including physical, social, learning, sensory, and psychological goals. Moreover, it has been reported that THR provides a multi-sensory experience, and improves motor functioning and sensory processing in children with ASD [19]. During the THR, the riding instructor and use of a therapeutic horse target specific needs of children with ASD. This is achieved through horsemanship skill instruction, such as holding a horse’s reins that provides a chance to train handgrip and arm strength of children with ASD, directly leading to stronger catching ability and greater throwing distance/performances [4,20–22]. Furthermore, the riders need to consciously control their own body movements and behavior on the horse and learn to adjust their body and postures to different positions (upright, prone, supine, forward, backward, and side-bending) while riding a horse. Additionally, the rhythmic movements of riding horses can stimulate the vestibular system, and a horse moving in a fixed rhythm may play a role in promoting calmness and body coordination [23,24].
Despite the growing interest in this area, it has been argued that there are is still insufficient evidence from methodologically rigorous studies to establish the solid link between THR program and motor skills of children with ASD [25]. Therefore, the purpose of this study was to determine the effects of a THR program on motor skills in children with ASD. Specifically, we hypothesized that children with ASD who participated in a 12-week THR program would experience improvements in motor skills as compared with children with ASD who were asked to maintain their normal lifestyle behaviors.
Participants were recruited as a part of large THR program at the International Equestrian Training Center AMR in Jinan, Shandong province of China. In the current study, sixty-eight children diagnosed with ASD aged from 5 to 10 years old were recruited from autism training centers, special schools, ASD therapeutic centers and online social media groups. Participants were asked to provide the official diagnostic document (indicating ASD criteria of the DSM-5) signed by the psychiatrist. Before the intervention, the participants were randomly divided into an experimental group and a wait-list control group. A simple randomization was conducted in which a parent of each ASD child asked to draw lots (even number = wait-list control; odd number = experimental group). A simple randomization was conducted in which a parent of each ASD child was asked to draw lots (even number = wait list control; odd number = experimental group).
The participants in the control group were given access to the intervention following completion of all the study procedures.
Thirty-four participants were assigned to the experimental group and thirty-four participants to the control group, for a total sample size of 68 children. Three children with ASD in the control group who completed baseline tests discontinued this program without any feedback. During the progress of the THR program, participants of the control group did not perform the interim-THR test (n = 3) and post-THR test (n = 1). With respect to the experimental group, before the interim-THR test, four children with ASD who either missed more than half of total training sessions (n = 3) or dropped out due to fear of horses (n = 1) were not finally included for this study. Furthermore, data were excluded if they were absent during the post-THR test day (n = 2) or discontinued the THR program through the interim-THR test to post-THR test (n = 2). In total, fifty-three participants (n = 53) who completed the current study were included (Fig. 1).
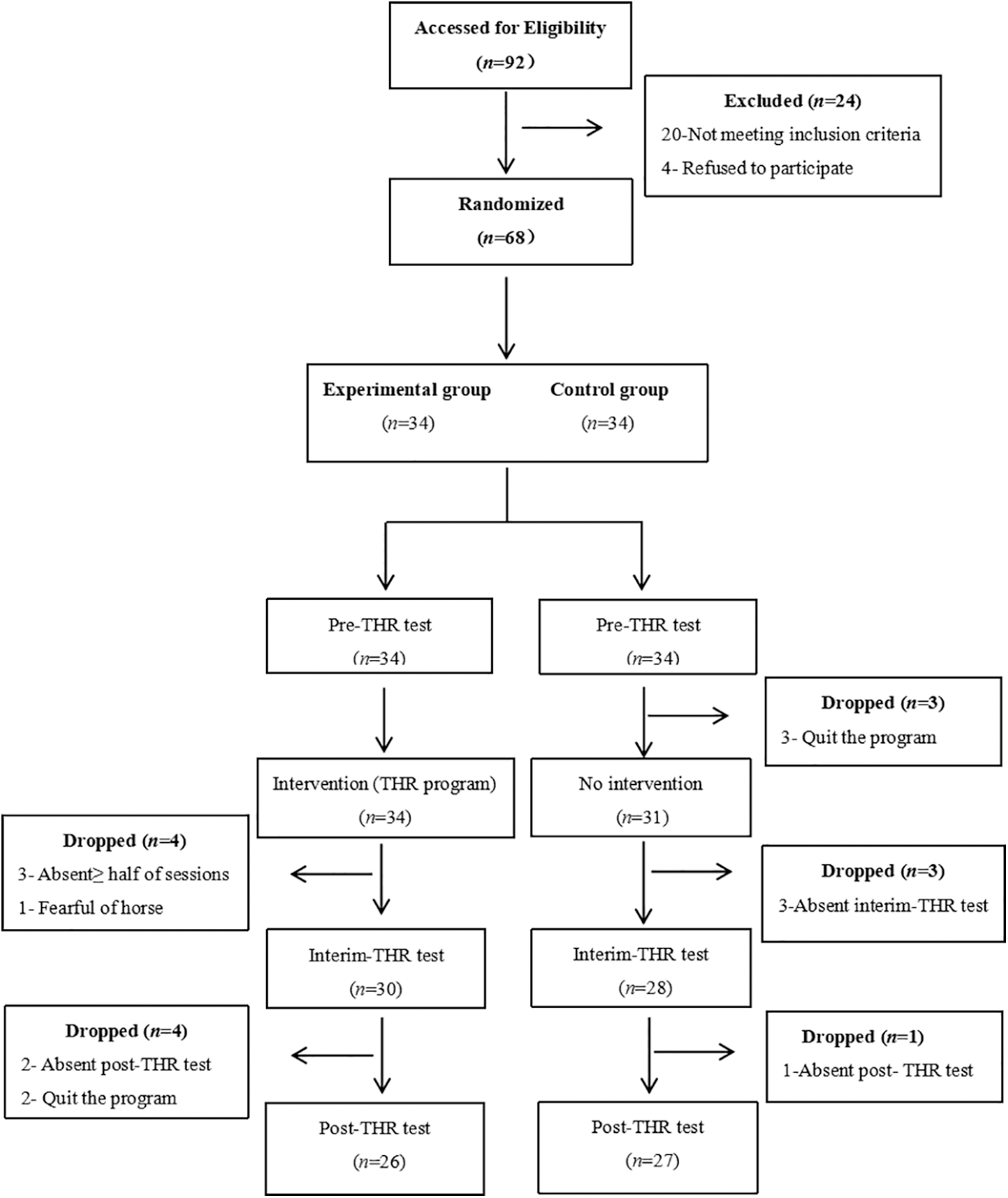
Figure 1: The flowchart of the participants
Independent sample t-tests and chi-square tests were employed to compare the baseline data between the experimental group and the control group. Results showed no significant differences between the two groups in regards to the participants’ age, gender, number of children in their family, parent employment and parent education level. The demographic data for the experimental group and control group are listed in Table 1 (all p > 0.05).

The study was approved by the Institutional Review Board of the first author’s institution (PN-2021-056). Written informed consent was obtained from parents before their children’s participation in THR program. A randomized controlled trial design was implemented to examine the effectiveness of a 12-week THR program on motor skills in children with ASD. The current study used a pre-THR test, interim-THR test and post-THR test to measure the possible changes in the motor skills of the participants at three time-intervals. The baseline assessment was conducted one week before the intervention, the interim-THR test was conducted at the 7th week, and post-THR test was conducted one week after the intervention.
The Test of Gross Motor Development-Third Edition (TGMD-3) [26] was utilized to assess motor skills, and it has two sub-skills including locomotor skill and ball skill. The locomotor skill test measures the gross motor skills that require fluid coordinated movements of the body as the child moves in one direction or another, and it contains six items (run, jump, hop, skip, gallop, and slide. The ball skill test measures the gross motor skills that demonstrate efficient throwing, striking, and catching movements, it includes several object control skills (catch, kick, strike with a bat, strike with a racquet, underarm throw, overhand throw, and dribble) that are each scored on several performance criteria, resulting in a raw score ranging between 0–100 (locomotor = 46, ball skills = 54) points. The TGMD-3 has demonstrated excellent reliability and validity evidence across the TGMD-3 intended age groups of 3–10 years old [26]. In the current study, the Cronbach’s alpha for the pre-THR test, interim-THR test and post-THR test on overall TGMD-3 were 0.890, 0.888 and 0.893, respectively.
The intervention was a THR program implemented at the International Equestrian Training Center AMR in Jinan, Shandong province of China. The THR program was conducted for 12 weeks, twice a week, with a total of 24 sessions. Each session lasted approximately 60 minutes, and it involved a cooperative therapeutic team which consists of horses, certified therapeutic riding instructors and trained volunteers. All THR sessions were conducted in small groups.
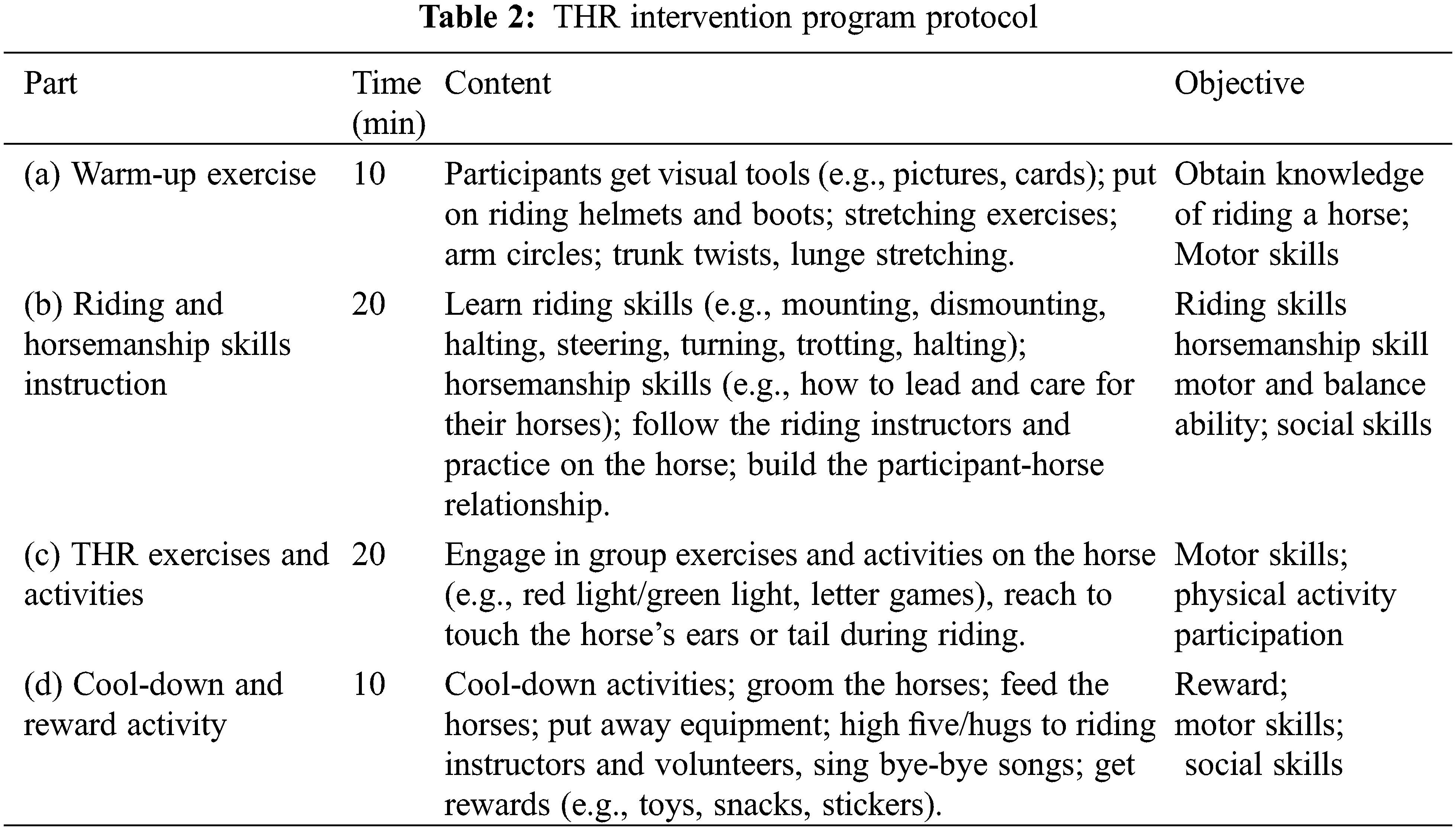
The contents of the THR session consisted of various structured activities that addressed motor skills improvement as well as horsemanship skills instruction. Each session followed a consistent routine during the 12-week intervention, which included (a) Warm-up activities; (b) Riding and horsemanship skills instruction; (c) THR exercises and activities; and (d) Cool-down and reward activity (Table 2). Picture schedules and visual aids (e.g., pictures, cards, colorful drawings) were used to facilitate the children’s understanding of what they would do and how to do it. Horses were assigned to children according to their height and weight. They were led by riding instructors and trained volunteers who worked with the same participant and the same horse throughout the program to promote relationship-building and maintain consistency. Before the intervention, all volunteers attended professional training and they played the role of “side walkers” during the THR. The riding instructors and volunteers closely monitored the children’s behavior to ensure their safety.
2.5 Data Collection and Analysis
The TGMD-3 was used to investigate the possible changes in motor skills at three time points, including pre-THR test, interim-THR test, and post-THR test. The TGMD-3 data were analyzed through repeated measures ANOVA at the three time-intervals to determine the differences in motor skills between the experimental group and the control group. Multivariate analysis results were used when the assumption of the sphericity test was not met, and within-subject effect test results were taken when the sphericity test was met. Significance was two-tailed at p < 0.05 and 0.01. Means and standard deviations at each time within each scale and sub-scale were calculated. All data analyses in the current study were performed using the Statistical Package for the Social Sciences (SPSS) version 25.0.
3.1 The Effects of the Therapeutic Horseback Riding Program on Overall TGMD-3
A series of ANOVAs were performed on the final sample to assess possible changes in motor skills in children with ASD who participated 12-week THR program as compared to children in the control group who continued as usual (Mauchly’s W = 0.858, p < 0.05). The results (Table 3) showed that there were significant differences in terms of time: F = 37.002, p < 0.01, and time x group interaction: F = 12.132, p < 0.01. The results of the multivariate tests showed that the time factor had significant effects (p < 0.01) on motor skills over time, and the interaction between testing intervals (pre-THR test, interim-THR test and post-THR test) and groups (experimental and control groups) also showed a statistically significant difference (p < 0.01). Therefore, the results illustrated that the motor skills of children with ASD in experimental group were significantly improved after the 12-week intervention.

Repeated ANOVA was conducted to assess motor skills in the experimental and control groups in the pre-THR test, interim-THR test, and post-THR test. As shown in Table 4, there was no significant difference between two groups before intervention (pre-THR test). In the experimental group, significant improvements were observed in two different time points (p < 0.01, pre-THR test to interim-THR test and interim-THR test to post-THR test), while a significant change only emerged from interim-THR test to post-THR test in the control group.

As shown in Fig. 2, participants in the experimental group experienced significant improvements in overall TGMD-3 score across time points, and there was a more noticeable improvement trend for the experimental group on overall scores compared to the control group, which indicated that the THR program intervention had a positive effect on motor skills in children with ASD in the experimental group.
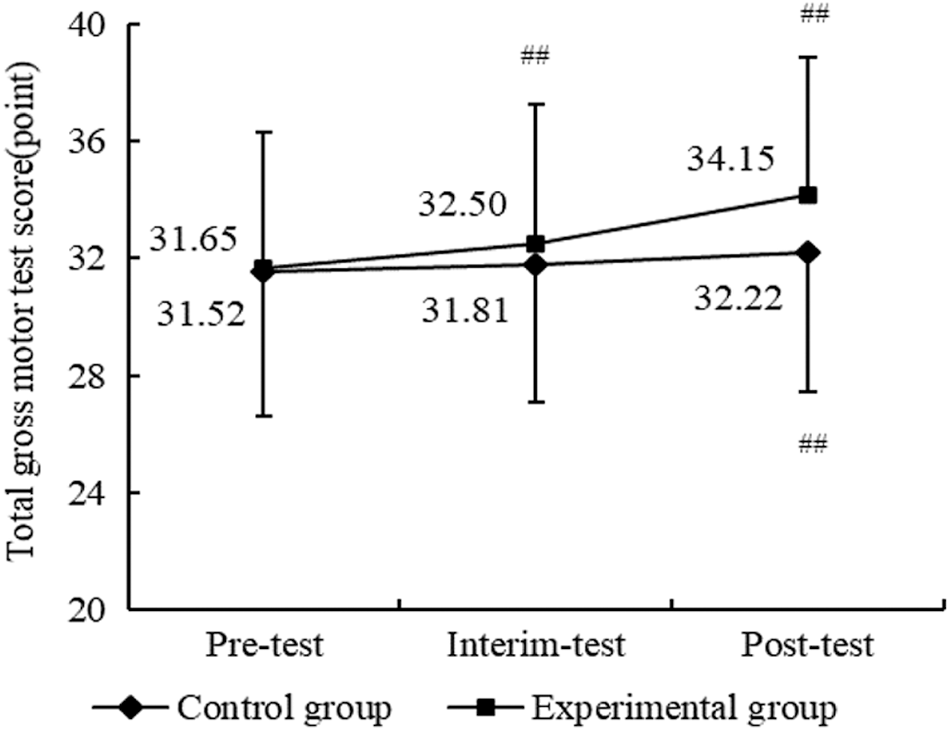
Figure 2: Results of THR program on motor skill between two groups. Compared with pre-test, ##p < 0.01
3.2 The Effects of the Therapeutic Horseback Riding Program on Sub-Tests of the TGMD-3
For the locomotor subtest, a series of ANOVAs were conducted to investigate the possible changes in children with ASD who participated in the 12-week THR program compared to children in the control group who continued as usual (Mauchly’s W = 0.891, p > 0.05). Within-Subjects effects showed that the time factor had a significant effect (F = 26.170, p < 0.01) and the time x group interaction also showed a statistically significant difference (F = 10.896, p < 0.01) (Table 5). Therefore, there were significant differences on the locomotor subtest between the two groups across the three time-points.
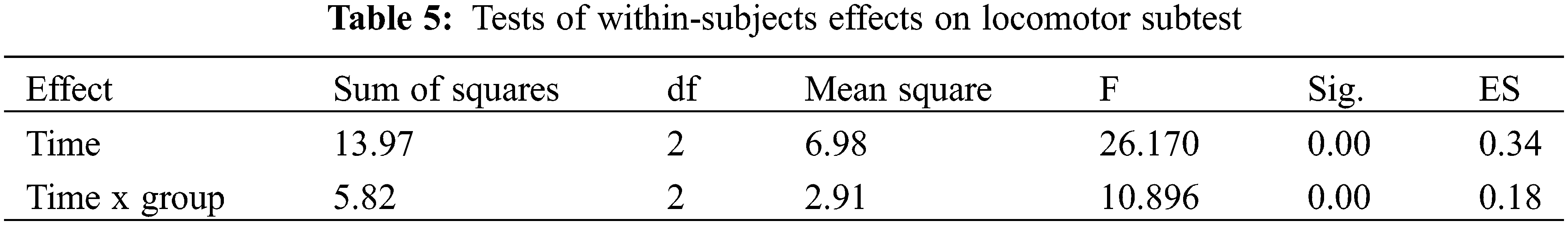
In relation to the between-group comparison of locomotor skills, as displayed in Table 6, there was no significant difference between the control group and experimental group across the three tests. However, as to within-group comparison, there were significant improvements in locomotor skills for the experimental group from pre-THR test to interim-THR test to post-THR test.
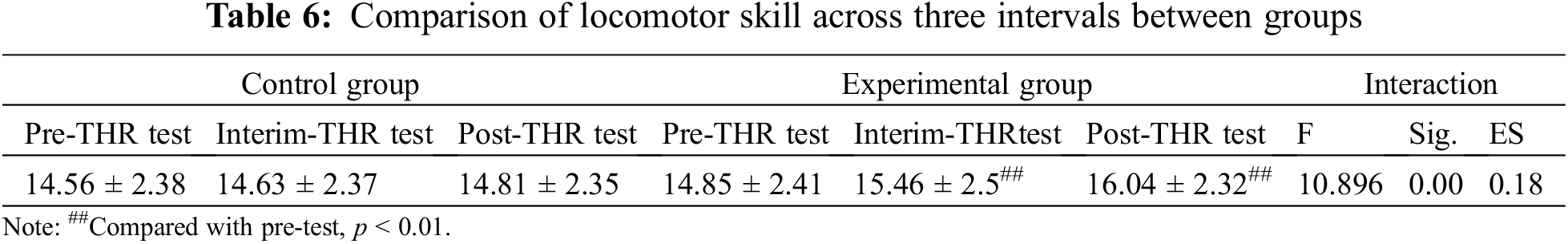
Fig. 3 displayed the improvement trends in locomotor skills for the experimental group and control group, respectively, and it showed that the experimental group exhibited a more noticeable improvement over time with significant differences. By comparison, no significant differences were found among the three tests in the control group.
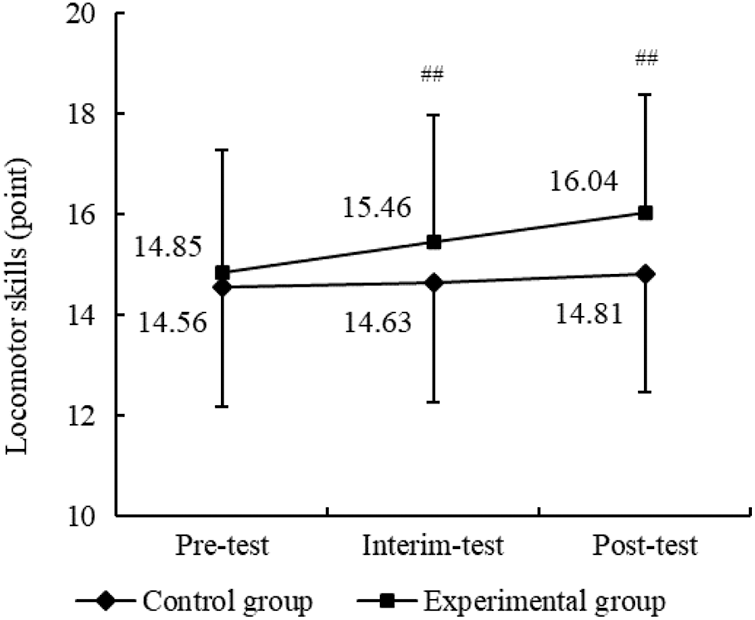
Figure 3: Results of THR program on locomotor skill between two groups. Notes: Compared with pre-test, ##p < 0.01
Follow-up ANOVAs were conducted to evaluate whether the THR program impacted the six items of locomotor skill between the two groups across three intervals. As shown in Table 7, there were no significant differences in all six items of locomotor skills for two groups across time points for the control group. However, the experimental group exhibited a more noticeable improvement in items of the run and gallop in interim-test and post-test (p < 0.01). Furthermore, in relation to between-group comparisons of the six items of locomotor skills, there were significant differences in run and gallop for the experimental group compared to the control group (p < 0.05) Fig. 4. Therefore, the results demonstrated the THR program significantly enhanced the run and gallop scores after 12-week of intervention.

Figure 4: (a–f) Results of the THR program on sub-domains of locomotor skill between two groups. Notes: Compared with pre-THR test, ##p < 0.01. Compared with the control group, *p < 0.05, **p < 0.01
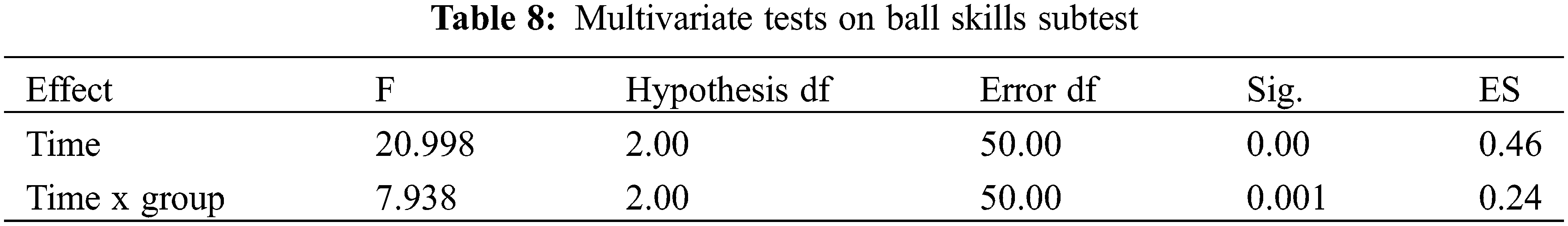
A series of ANOVAs were conducted to evaluate the possible changes in the ball skills subtest in the participants of the experimental group as compared to those in the control group (Mauchly’s W = 0.769, p < 0.05). As displayed in Table 8, Multivariate test results showed that the main effect of time was statistically significant (F = 20.998, p < 0.01), and the time x group interaction also showed a statistically significant difference (F = 7.938, p < 0.01) (Table 8). Therefore, there were significant differences in ball skills between the two groups across time.
As shown in Table 9, in relation to between-group comparison of ball skills, there was no significant difference between the control group and experimental groups across time. However, as to within-group comparison, after the 12-week THR program intervention, there were significant differences from pre-test to post-test for both groups. However, as shown in Fig. 5, there was a greater improvement in ball skills for the experimental group compared to the control group (p < 0.01).
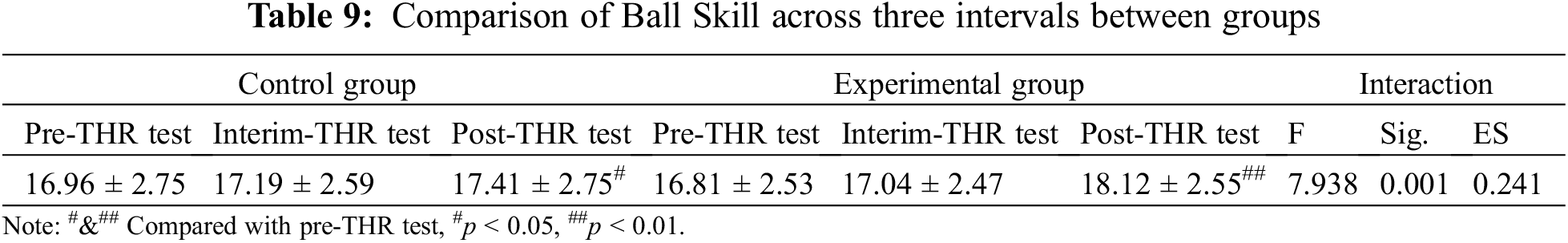
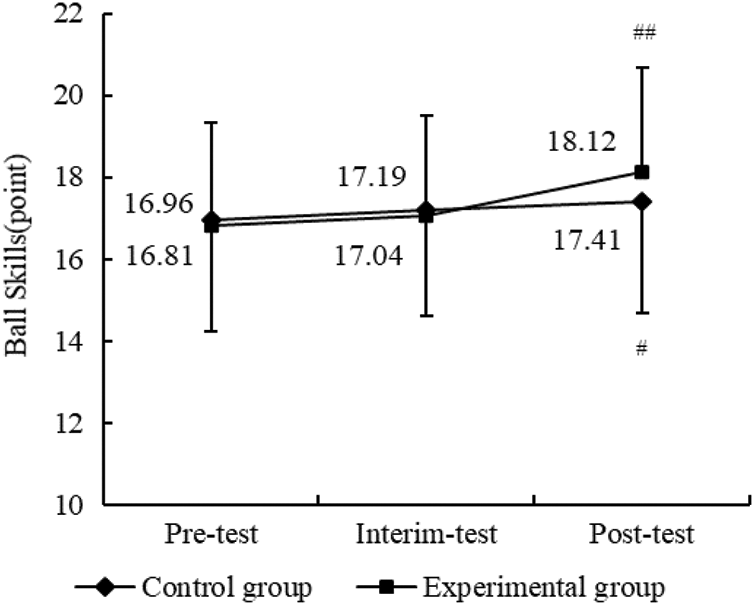
Figure 5: Results of THR program on ball skill between two groups. Compared with pre-THR test, #p < 0.05, ##p < 0.01
Follow-up ANOVAs were conducted to examine the effects of the THR program on the seven subdomains of ball skills between the two groups across three intervals. As shown in Table 10, greater improvements were observed in the two-hand strike of a stationary ball, two-hand catch and kicking a stationary ball in the experimental group over time (p < 0.01). These results implied that the 12-week intervention significantly enhanced the skills of the two-hand strike of a stationary ball, two-hand catch and stationary ball kick. Moreover, there were significant differences in the two-hand catch for the experimental group over time compared to the control group (p < 0.01).

Motor dysfunction or deficits have been increasingly recognized among children with ASD, which is linked to reduced opportunity of participating in exercise as well as increased level of social isolation and anxiety [6,17,27]. Against this background, this study aimed to determine the effects of a 12-week THR program on motor skills among children with ASD. To our knowledge, it is the first study exploring the effectiveness of a THR program on motor skills utilizing a randomized controlled trial (RCT) design. Therefore, this study addresses some limitations of previous studies, with its relatively larger sample size, experimental design, and very specific choice of interventions targeting motor skills [28].
Results indicate that children with ASD who participated in the 12-week THR program demonstrated significantly better motor skills throughout the intervention period, while participants in the control group only reported significant improvement from interim-THR test to post-THR test. Such findings are consistent with some previous studies with either small sample size or no control group indicating that THR program effectively improved motor skills of children with ASD [18,29–32]. With respect to sub-skills, significant changes in the experimental group were observed in the items of run, gallop and two-hand catch as measured by the TGMD-3 when compared to the control group.
The possible mechanisms underlying the benefit of THR for motor skills development warrant discussion. First, the THR program provides the participants the unique opportunity to improve stability, balance and postural control. Specifically, horses take approximately 100 steps per minute while walking [33] and each step the horse took has potentially challenged riders to stabilize their center of weight. Each THR session lasted 40 minutes with roughly 4,000 steps. As a result, the participants repeatedly respond to the variability in the horse’s movement to maintain their stability, postural control and then keep balance constantly [34] which all contribute to motor skills improvement or balance-related performance like run and gallop. Secondly, the THR-motor skill relationship is related to sensory integration, a key precursor to efficient motor movements. The majority of children with ASD experience difficulty in integrating their senses and understanding how their bodies relate to the external world. THR is a beneficial way to help them gain a sense of body-awareness while improving sensory integration and control of their bodies. Because being on a horse is a sensation-rich experience, children with ASD can benefit from the integration of their motor, visual, auditory, olfactory, and tactile senses [35]. The process of horseback riding generates strong sensory stimulation to muscles and joints, leading to better catching and balance-related performances.
In addition to the mechanism discussed above, several program-level features contributed to the success of the current study’s intervention. Specifically, the combination of the structured contents in each session, well-trained horses, professional therapeutic riding instructors, trained, qualified volunteers, and visual supplementary tools (e.g., pictures, cards, colorful drawings), which forms a cooperative therapeutic team and strategy which supports the effectiveness of the THR program. Involvement in THR has the potential to enable children with ASD to activate their automatic postural mechanisms to improve stability while riding the horses and performing functional tasks on the horses. During the THR session, participants sit on the horses while therapeutic riding instructors guide the horses’ movements which stimulates the development of neural connections in children’s brains that may help with motor development. Following the horse’s movements could help the children with ASD facilitate a range of abilities from muscular coordination to respiratory control and attention skills. Additionally, children with ASD could create an emotional bond with the horse and the therapeutic riding instructor that encourages them to perform various skill-building tasks during riding a horse. Through different types of riding activities, the therapists provide the optimal sensory and neurological input on the corresponding participant, and then analyze the participants’ responses and adjust the treatment while riding. Therefore, the providers of both horses and therapeutic riding instructors could maximize treatment effects on children with ASD by prompting the participants to build connections during horseback riding to strengthen the bond between the children and the horses.
The limitations of the present study must be acknowledged. There were no significant enhancements in other sub-skills, which may suggest that a longer treatment program is needed to see the progress. Also, further research is warranted to examine the potential influence of THR on motor performance based on a longer and higher intensity version of the program. A longer treatment time (>12 weeks) or more frequent sessions (e.g., 3–4/week) in the THR program may result in greater or more measurable benefits for children with ASD. In addition, the potential longer-term influences of the intervention (e.g., 6 months measurement after THR program intervention) should be measured in future studies to examine whether the effects are maintained over time. Secondly, between-group differences on overall motor skills were not observed in the present study, whereas significant change (from interim-THR test to post-THR test) in the control group emerged. Such interesting findings may be attributed to physical activity level, but this key lifestyle behavior was not objectively (actigraphy) and subjectively (parental questionnaire) monitored in the present study. Thirdly, the testing environment may cause worse performance as children with ASD may not be as cooperative as typically developing children. Thus, process-based assessment or observation in this context could be conducted through recording game-based learning.
In conclusion, these results suggest that participation in THR is an effective therapeutic method for children with ASD. Furthermore, the results provide evidence base supporting THR as a therapeutic treatment to improve motor skills for children with ASD. While there is still potential for further development of the THR program and for testing long-term effects, the clinical implication is that THR could be an effective complementary therapy in their treatment to target motor difficulties, which are experienced by many children with ASD.
Authorship: Conceptualization, M.Z.; Data curation, J.L.; Formal analysis, Y.Y.; Funding acquisition, M.Z. and L.Z.; Methodology, J.L.; Software, Y.Y. and Z.Z.; Supervision, A.T. and L.Z.; Validation, L.L.; Visualization, S.H. and A.T.; Writing – original draft, M.Z. and L.Z.; Writing–review & editing, S.H. and A.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Shenzhen Univseristy (PN-2021-056).
Funding Statement: This research was funded by Guangdong Planning Office of Philosophy and Social Science (General Project in 2021, No. GD21CTY02) and Shenzhen University Young Teachers Research Initiation Project (No. 20210402). This study is supported by Start-Up Research Grant of Shenzhen University (20200807163056003) and Start-Up Research Grant (Peacock Plan: 20191105534C).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (2013). Arlington, VA, American Psychiatric Association. [Google Scholar]
2. Maenner, M. J., Shaw, K. A., Bakian, A. V., Bilder, D. A., Durkin, M. S. et al. (2021). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2018. Surveillance Summaries, 70(11), 1–16. DOI 10.15585/mmwr.ss7011a1. [Google Scholar] [CrossRef]
3. Trzmiel, T., Purandare, B., Michalak, M., Zasadzka, E., Pawlaczyk, M. (2019). Equine assisted activities and therapies in children with autism spectrum disorder: A systematic review and a meta-analysis. Complementary Therapies in Medicine, 42(4), 104–113. DOI 10.1016/j.ctim.2018.11.004. [Google Scholar] [CrossRef]
4. Zoccante, L., Marconi, M., Ciceri, M. L., Gagliardoni, S., Gozzi, L. A. et al. (2021). Effectiveness of equine-assisted activities and therapies for improving adaptive behavior and motor function in Autism Spectrum Disorder. Journal of Clinical Medicine, 10(8), 1726. DOI 10.3390/jcm10081726. [Google Scholar] [CrossRef]
5. Alsaedi, R. H. (2020). An assessment of the motor performance skills of children with autism spectrum disorder in the gulf region. Brain Sciences, 10(9), 607. DOI 10.3390/brainsci10090607. [Google Scholar] [CrossRef]
6. Crucitti, J., Hyde, C., Stokes, M. A. (2020). Hammering that Nail: Varied praxis motor skills in younger autistic children. Journal of Autism and Developmental Disorders, 50(9), 3253–3262. DOI 10.1007/s10803-019-04136-4. [Google Scholar] [CrossRef]
7. Landa, R., Garrett-Mayer, E. (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 629–638. DOI 10.1111/j.1469-7610.2006.01531.x. [Google Scholar] [CrossRef]
8. Lane, A., Harpster, K., Heathcock, J. (2012). Motor characteristics of young children referred for possible autism spectrum disorder. Pediatric Physical Therapy, 24, 21–29. DOI 10.1097/PEP.0b013e31823e071a. [Google Scholar] [CrossRef]
9. Liu, T., Breslin, C. (2013). Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Research in Autism Spectrum Disorders, 7(10), 1244–1249. DOI 10.1016/j.rasd.2013.07.002. [Google Scholar] [CrossRef]
10. Lloyd, M., MacDonald, M., Lord, C. (2013). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. DOI 10.1177/1362361311402230. [Google Scholar] [CrossRef]
11. Matson, J., Mahan, S., Fodstad, J., Hess, J., Neal, D. (2010). Motor skill abilities in toddlers with autistic disorder, pervasive developmental disorder-not otherwise specified, and atypical development. Research in Autism Spectrum Disorders, 4, 444–449. DOI 10.1016/j.rasd.2009.10.018. [Google Scholar] [CrossRef]
12. Ozonoff, S., Young, G. S., Goldring, S., Greiss-Hess, L., Herrera, A. M. et al. (2008). Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders, 38(4), 644–656. DOI 10.1007/s10803-007-0430-0. [Google Scholar] [CrossRef]
13. Bhat, A. N., Galloway, J. C., Landa, R. J. (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development, 35(4), 838–846. DOI 10.1016/j.infbeh.2012.07.019. [Google Scholar] [CrossRef]
14. Chawarska, K., Paul, R., Klin, A., Hannigen, S., Dichtel, L. E. et al. (2007). Parental recognition of developmental problems in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(1), 62–72. DOI 10.1007/s10803-006-0330-8. [Google Scholar] [CrossRef]
15. Flanagan, J. E., Landa, R., Bhat, A., Bauman, M. (2012). Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy, 66(5), 577–585. DOI 10.5014/ajot.2012.004192. [Google Scholar] [CrossRef]
16. Cosbey, J., Johnston, S. S., Dunn, M. L. (2010). Sensory processing disorders and social participation. American Journal of Occupational Therapy, 64(3), 462–473. DOI 10.5014/ajot.2010.09076. [Google Scholar] [CrossRef]
17. Hilton, C. L., Zhang, Y., Whilte, M. R., Klohr, C. L., Constantino, J. (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. DOI 10.1177/1362361311423018. [Google Scholar] [CrossRef]
18. Lanning, B. A., Baier, M. E. M., Ivey-Hatz, J., Krenek, N., Tubbs, J. D. (2014). Effects of equine assisted activities on autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(8), 1897–1907. DOI 10.1007/s10803-014-2062-5. [Google Scholar] [CrossRef]
19. Kashefimehr, B., Huri, M., Kayihan, H., Havaei, N. (2021). The relationship between the sensory processing and occupational motor skills of children with autism spectrum disorder. International Journal of Therapy and Rehabilitation, 28(4), 1–8. DOI 10.12968/ijtr.2019.0137. [Google Scholar] [CrossRef]
20. Bertoti, D. B. (1988). Effect of therapeutic horseback riding on posture in children with cerebral palsy. Physical Therapy, 68(10), 1505–1512. DOI 10.1093/ptj/68.10.1505. [Google Scholar] [CrossRef]
21. Bracher, M. (2000). Therapeutic horse riding: What has this to do with occupational therapists? British Journal of Occupational Therapy, 63(6), 277–282. DOI 10.1177/030802260006300606. [Google Scholar] [CrossRef]
22. Snider, L., Korner-Bitensky, N., Kammann, C., Warner, S., Saleh, M. (2007). Horseback riding as therapy for children with cerebral palsy: Is there evidence of its effectiveness? Physical & Occupational Therapy in Pediatrics, 27(2), 5–23. DOI 10.1080/J006v27n02_02. [Google Scholar] [CrossRef]
23. Ward, S. C., Whalon, K., Rusnak, K., Wendell, K., Paschall, N. (2013). The association between therapeutic horseback riding and the social communication and sensory reactions of children with autism. Journal of Autism and Developmental Disorders, 43(9), 2190–2198. DOI 10.1007/s10803-013-1773-3. [Google Scholar] [CrossRef]
24. Zhao, M., Chen, S., You, Y., Wang, Y., Zhang, Y. (2021). Effects of a therapeutic horseback riding program on social interaction and communication in children with autism. International Journal of Environmental Research and Public Health, 18(5), 2656. DOI 10.3390/ijerph18052656. [Google Scholar] [CrossRef]
25. Nieforth, L. O., Schwichtenberg, A. J., O’Haire, M. E. (2021). Animal-assisted interventions for autism spectrum disorder: A systematic review of the literature from 2016 to 2020. Review Journal of Autism and Developmental Disorders, 46(10), 3344. DOI 10.1007/s40489-021-00291-6. [Google Scholar] [CrossRef]
26. Webster, K., Ulrich, D. (2017). Evaluation of the psychometric properties of the test of gross motor development—Third edition. Journal of Motor Learning and Development, 5(1), 45–58. DOI 10.1123/jmld.2016-0003. [Google Scholar] [CrossRef]
27. Ruggeri, A., Dancel, A., Johnson, R., Sargent, B. (2020). The effect of motor and physical activity intervention on motor outcomes of children with autism spectrum disorder: A systematic review. Autism, 24(3), 544–568. DOI 10.1177/1362361319885215. [Google Scholar] [CrossRef]
28. Caçola, P. (2016). Physical and mental health of children with developmental coordination disorder. Frontiers in Public Health, 4, 224. DOI 10.3389/fpubh.2016.00224. [Google Scholar] [CrossRef]
29. Gabriels, R. L., Pan, Z., Dechant, B., Agnew, J. A., Brim, N. et al. (2015). Randomized controlled trial of therapeutic horseback riding in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(7), 541–549. DOI 10.1016/j.jaac.2015.04.007. [Google Scholar] [CrossRef]
30. Holm, M. B., Baird, J. M., Kim, Y. J., Rajora, K. B., D’Silva, D. et al. (2014). Therapeutic horseback riding outcomes of parent-identified goals for children with autism spectrum disorder: An ABA’ multiple case design examining dosing and generalization to the home and community. Journal of Autism and Developmental Disorders, 44(4), 937–947. DOI 10.1007/s10803-013-1949-x. [Google Scholar] [CrossRef]
31. Steiner, A. M., Koegel, L. K., Koege, R. L., Ence, W. A. (2012). Issues and theoretical constructs regarding parent education for autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(6), 1218–1227. DOI 10.1007/s10803-011-1194-0. [Google Scholar] [CrossRef]
32. Wuang, Y. P., Wang, C. C., Huang, M. H., Su, C. Y. (2010). The effectiveness of simulated developmental horse-riding program in children with autism. Adapted Physical Activity Quarterly, 27(2), 113–126. DOI 10.1123/apaq.27.2.113. [Google Scholar] [CrossRef]
33. Clayton, H. M., Hoyt, D. F., Wickler, S. J., Cogger, E. A., Lanovaz, J. L. (2002). Hindlimb net joint energies during swing phase as a function of trotting velocity. Equine Veterinary Journal Supplement, 34(S34), 363–367. DOI 10.1111/j.2042-3306.2002.tb05449.x. [Google Scholar] [CrossRef]
34. Shurtleff, T. L., Standeven, J. W., Engsberg, J. R. (2009). Changes in dynamic trunk/head stability and functional reach after hippotherapy. Archives of Physical Medicine and Rehabilitation, 90(7), 1185–1195. DOI 10.1016/j.apmr.2009.01.026. [Google Scholar] [CrossRef]
35. Hippotherapy for Autistic Children (2021). https://hiddentalentsaba.com/horse-therapy-for-autism/. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |