 Open Access
Open Access
ARTICLE
Computational Investigation of Brownian Motion and Thermophoresis Effect on Blood-Based Casson Nanofluid on a Non-linearly Stretching Sheet with Ohmic and Viscous Dissipation Effects
1 Department of Applied Mathematics, M. J. P. Rohilkhand University, Bareilly, 243006, Uttar Pradesh, India
2 Fakulti Teknologi dan Kejuruteraan Mekanikal, Universiti Teknikal Malaysia, Melaka, Durian Tunggal, 76100, Malaysia
3 Forecasting and Engineering Technology Analysis (FETA) Research Group, Universiti Teknikal Malaysia, Melaka, Durian Tunggal, 76100, Malaysia
* Corresponding Author: Nurul Amira Zainal. Email:
Computer Modeling in Engineering & Sciences 2024, 141(2), 1137-1163. https://doi.org/10.32604/cmes.2024.055493
Received 28 June 2024; Accepted 20 August 2024; Issue published 27 September 2024
Abstract
Motivated by the widespread applications of nanofluids, a nanofluid model is proposed which focuses on uniform magnetohydrodynamic (MHD) boundary layer flow over a non-linear stretching sheet, incorporating the Casson model for blood-based nanofluid while accounting for viscous and Ohmic dissipation effects under the cases of Constant Surface Temperature (CST) and Prescribed Surface Temperature (PST). The study employs a two-phase model for the nanofluid, coupled with thermophoresis and Brownian motion, to analyze the effects of key fluid parameters such as thermophoresis, Brownian motion, slip velocity, Schmidt number, Eckert number, magnetic parameter, and non-linear stretching parameter on the velocity, concentration, and temperature profiles of the nanofluid. The proposed model is novel as it simultaneously considers the impact of thermophoresis and Brownian motion, along with Ohmic and viscous dissipation effects, in both CST and PST scenarios for blood-based Casson nanofluid. The numerical technique built into MATLAB’s bvp4c module is utilized to solve the governing system of coupled differential equations, revealing that the concentration of nanoparticles decreases with increasing thermophoresis and Brownian motion parameters while the temperature of the nanofluid increases. Additionally, a higher Eckert number is found to reduce the nanofluid temperature. A comparative analysis between CST and PST scenarios is also undertaken, which highlights the significant influence of these factors on the fluid’s characteristics. The findings have potential applications in biomedical processes to enhance fluid velocity and heat transfer rates, ultimately improving patient outcomes.Graphic Abstract
Keywords
Nomenclature
| Suction parameter (-) | |
| Slip parameter (-) | |
| Scaled similarity parameter (-) | |
| Scaled concentration (-) | |
| Dimensionless temperature (-) | |
| Fluid viscosity coefficient (kgm/s) | |
| Cauchy stress tensor (N/m2) | |
| Dynamic fluid viscosity (N/s2) | |
| Density of fluid (kg/m3) | |
| Density of nanofluid (kg/m3) | |
| Thermal diffusivity (m2/s) | |
| Thermal capacitance of nanofluid (J/K) | |
| Thermal capacitance of fluid (J/K) | |
| Drag coefficient (-) | |
| Heat transfer coefficient (W/m2K) | |
| Local nanoparticles volume ratio (mol/m3) | |
| Temperature far away from sheet (K) | |
| Heat-induced migration coefficient (-) | |
| Particle diffusivity (-) | |
| Dimensionless potential function (-) | |
| Deformation velocity of sheet (m/s) | |
| Uniform temperature across stretching sheet (K) | |
| Nanoparticle abundance on stretched surface (mol/m3) | |
| Constants (-) | |
| Parameter of surface temperature (-) | |
| Local Sherwood number (-) | |
| Local Nusselt number (-) | |
| Local skin friction coefficient (-) | |
| Thermal migration coefficient (-) | |
| Brownian diffusion coefficient (-) | |
| Mass transport number (-) | |
| Thermal efficiency index (-) | |
| Magnetic flux density (-) | |
| Prandtl number (-) | |
| Pressure of the fluid (kg/ms2) | |
| Casson fluid component (-) | |
| Fluid thermal state (K) | |
| Transverse and longitudinal velocity (m/s) | |
| Local thermal energy transfer (J/s) | |
| Local mass transport rate (m/s) | |
| Wall shear stress (N/m2) |
Blood flow is the dynamic movement of blood throughout the circulatory system, vital for supplying nutrients and oxygen to tissues and organs while removing byproducts [1]. However, disruptions in blood flow can lead to a range of associated diseases and health complications. Conditions such as hypertension, atherosclerosis, and thrombosis can impair blood flow by narrowing blood vessels, increasing clot formation, or raising blood pressure [2]. These diseases can have serious consequences, including heart attacks, strokes, and organ damage. Thus, maintaining proper control over blood flow is crucial for overall health and well-being. Control mechanisms, such as regulation of blood pressure, vascular tone, and clotting factors, play a pivotal role in ensuring ample blood perfusion to fulfill the body’s metabolic needs. A better understanding of blood flow control is essential for preventing and managing cardiovascular diseases and optimizing health outcomes [3]. Additionally, advancements in medical technology and therapies aimed at improving blood flow regulation continue to be a focus of research and clinical practice. The Casson fluid model [4] is a rheological model commonly used to describe the fluidic behavior of multiphase fluids, particularly those with non-Newtonian characteristics. This model extends beyond the simple Newtonian fluid model by considering the presence of yield stress below which the fluid does not flow. Instead, it behaves like a solid until a certain threshold of stress is exceeded, at which point it transitions to a fluid-like behavior with a constant viscosity. The Casson fluid model is particularly useful in describing the flow of materials such as paints, gels, and biological fluids like blood, which exhibit non-Newtonian behavior. Blood is regarded as a Casson fluid due to its unique rheological properties [5]. Unlike simple Newtonian fluids, blood displays a nonlinear relationship between shear stress and shear rate, with its viscosity varying depending on factors such as shear rate, temperature, and hematocrit levels. Additionally, blood exhibits a yield stress, requiring a certain amount of force to initiate flow. Below this threshold, blood behaves as a non-flowing, structured fluid akin to a solid, while above it, it flows as a viscous liquid. This behavior is crucial for understanding blood flow dynamics in the circulatory system, where the Casson fluid model provides a more accurate representation compared to traditional Newtonian models. By regarding blood as a Casson fluid, researchers and clinicians can better interpret hemodynamic parameters, design medical devices, and develop computational models to simulate blood flow in health and disease [6]. On the other hand, nanotechnology, a cutting-edge field at the intersection of science, engineering, and technology, focuses on the manipulation of matter at the nanoscale, typically ranging from 1 to 100 nanometers. Its importance lies in its potential to revolutionize various industries and address pressing global challenges. Mauter et al. [7] and Wiek et al. [8] discussed the role of nanotechnology in global challenges for sustainable development. In medicine, nanotechnology offers promising avenues for targeted drug delivery, early disease detection, and precise imaging techniques, thus improving treatment efficacy while minimizing side effects [9]. Qi et al. [10] conducted an experimental investigation focusing on nanofluids’ flow behavior and heat transfer properties within double-tube heat exchangers, emphasizing thermal efficiency. Nanostructured particles, when engineered appropriately and dispersed in blood, can modulate various aspects of blood flow, including viscosity, rheology, and clotting behavior. Moreover, nanofluids containing functionalized nanoparticles can be advanced contrast agents for medical imaging techniques [11], enabling high-resolution visualization of blood vessels and tissue perfusion.
Brownian motion and thermophoresis effects play crucial roles in the behavior of nanoparticles within blood flow, presenting both challenges and opportunities in biomedical applications [12]. Brownian motion [13] refers to the random motion of particles suspended in a fluid due to collisions with surrounding molecules. In blood flow, nanoparticles experience Brownian motion, which affects their dispersion, distribution, and interaction with cells and tissues. Thermophoresis [14], on the other hand, is the migration of particles in response to temperature gradients within a fluid. In blood flow, thermophoresis effects can arise due to variations in temperature along blood vessels, influencing the movement of nanoparticles. Understanding and controlling these phenomena is essential for designing effective nanoparticle-based therapies and diagnostic tools. The importance of considering Brownian motion and thermophoresis effects lies in their impact on the targeting, delivery, and retention of nanoparticles in specific tissues or organs [15]. Zuberi et al. [16] investigated the effect of Brownian motion and thermophoresis on blood-based Casson nanofluid. Researchers can develop nanoparticle formulations with enhanced circulation times, improved targeting efficiency, and reduced off-target effects, thereby advancing precision medicine and personalized healthcare by utilizing these effects. Recently, Madhura et al. [17] conducted a numerical study on magnetohydrodynamics (MHD) Carreau nanofluid with thermophoresis and Brownian motion effects. In blood flow with nanoparticles, viscous and Ohmic dissipation effects [18] play significant roles, impacting the overall dynamics and efficiency of circulation. Viscous dissipation occurs due to the internal friction between blood components and the vessel walls as the fluid moves, leading to the conversion of mechanical energy into heat. Tang et al. [19] investigated the characteristics of blood flow using Au-nanofluid in a stenotic artery with porous walls and accounting for the viscous dissipation effect. This dissipation affects the flow velocity profile, pressure distribution, and energy expenditure within the circulatory system. On the other hand, Ohmic dissipation arises from the electrical resistance encountered by nanoparticles in blood, particularly metallic nanoparticles, when exposed to electromagnetic fields or currents [20]. These effects contribute to the heating of nanoparticles, potentially influencing their behavior, distribution, and therapeutic efficacy. The understanding and quantification of these dissipation mechanisms are essential for optimizing nanoparticle-based therapies and diagnostic techniques in blood flow applications. Under Ohmic heating, Yusuf explored the study of entropy generation for the convective flow of unsteady MHD flow over a vertical stretching sheet [21]. Siddiqui et al. [22] explored the film flow of nano-micropolar fluids, taking into account dissipation effects. By undertaking viscous and Ohmic dissipation effects, researchers can refine nanoparticle formulations, design efficient delivery strategies, and enhance treatment outcomes while minimizing unwanted side effects, thereby advancing precision medicine and personalized healthcare [23,24].
Fluid flow over-stretching sheets represent a class of fundamental problems in fluid mechanics with diverse applications in engineering and industrial processes. These problems involve the study of flow dynamics over a solid surface that is continuously stretched or contracted [25,26]. Cortell [27] investigated the 2-dimensional boundary layer flow of Newtonian fluid across a stretching surface, a pioneering inquiry that spurred considerable attention toward the flow of fluid on stretching surfaces. Researchers such as Alahmadi et al. [28], Raza et al. [29], Pandey et al. [30], and Boujelbene et al. [31] have explored various theoretical and computational models to elucidate slip flow phenomena. An interesting study conducted by Reddy et al. [32] is the “Computational investigation of chemical reaction and thermal diffusion Brinkman flow over an oscillating absorbent plate.” The modeling of blood flow over-stretching sheets enables researchers to gain valuable insights into the mechanics of cardiovascular conditions such as atherosclerosis, aneurysms, and arterial stenosis.
The investigation of blood flow over a stretching sheet with constant surface temperature or particular surface temperature, particularly in the presence of nanoparticles, holds significant importance in biomedical engineering and healthcare applications [33]. A better understanding of behavior of blood over such surfaces under different temperature conditions is crucial for optimizing various medical procedures and devices. For instance, in hyperthermia treatments for cancer therapy, maintaining a constant surface temperature on the stretching sheet can help precisely control the heating of nanoparticles within the blood, enhancing the effectiveness of localized tumor treatment while minimizing damage to healthy tissues [34–36]. Similarly, in diagnostic applications such as thermal imaging for detecting vascular abnormalities, controlling the surface temperature of the stretching sheet enables accurate interpretation of temperature distributions within the blood vessels, aiding in the early detection of diseases. Yazdi et al. [37] and Sk et al. [38] have explored the slip flow and heat transfer over non-linear permeable stretching surfaces, considering chemical reactions and magnetic field. These investigations have revealed that the slip velocity and temperature profiles are significantly influenced by the stretching parameter, chemical reaction rate, and magnetic field strength. Further, numerical investigations by Rana et al. [39] and Qayyum et al. [40] have been conducted to examine the flow and heat transfer of nanofluids over nonlinearly stretching sheets. These studies have demonstrated that the nanofluid velocity and temperature profiles are influenced by the stretching parameter, nanoparticle volume fraction, and viscous dissipation. Chaudhary et al. [41] have investigated the MHD blood-nanofluid flow through non-linearly stretched sheet, taking into account heat generation and permeable media. The heat transfer analysis of Radiative-Marangoni convective flow in nanofluids has been examined by Zari et al. [42] considering Lorentz force and porosity effect. Recently, Hou et al. [43] investigated the application of a hybrid model and ferrofluids in fluid flow and heat transfer using the finite element method. Makkar et al. [44] numerically investigated the MHD flow of fluid with double diffusive effects along with Ohmic and viscous dissipation. The impact of Brownian motion and thermophoresis for thermal and chemically reacting nanofluid (Casson) has been explored by Tawade et al. [45]. Researchers can advance the influence of Brownian motion and thermophoresis for improving drug delivery, ultimately enhancing patient outcomes [46–49]. The motivation for the current study stems from the critical need to comprehensively understand the intricate dynamics of blood flow with nanoparticles in physiological conditions. Blood is a complex non-Newtonian fluid, and incorporating nanoparticles further complicates its behavior. This pioneering study bridges a critical research gap by meticulously incorporating Ohmic and viscous dissipation effects in the blood-based Casson nanofluid flow analysis over a non-linearly stretching surface. The omission of these crucial factors in previous studies has led to a significant knowledge deficit, as Ohmic dissipation plays a vital role in regulating heat transfer processes. In contrast, viscous dissipation influences the fluid’s rheological behavior, thereby affecting the overall dynamics of blood flow. By accounting for these effects, this study provides a more comprehensive understanding of the complex interactions between nanoparticles and blood, ultimately enabling the optimization of nanoparticle-based therapies and medical devices. Notably, this investigation exhibits a dual nature, as it is employed in both Constant Surface Temperature (CST) and Prescribed Surface Temperature (PST) cases, thereby offering a more nuanced appreciation of the intricate relationships between temperature, fluid dynamics, and heat transfer in blood-based nanofluids. The novelty of this study lies in its thorough examination of the interplay between Ohmic and viscous dissipation, Brownian motion, and thermophoresis in blood-based Casson nanofluids for two different cases of CST and PST, a topic that has not been explored previously. To the best of our knowledge, no such study has been conducted yet, making this research a groundbreaking contribution to the field of biomedical engineering and nanofluid dynamics. The governing partial differential equations are transformed into nonlinear ordinary differential equations via similarity transformations, enabling numerical solutions using MATLAB’s bvp4c module. This framework holds promise for regulating heat transfer processes in designing biomedical devices, targeted drug delivery, treatment of tumors by hyperthermia and treatment of other diseases.
The present study delves into the dynamics of Casson nanofluid flow adjacent to a stretching sheet, characterized by a velocity profile denoted by
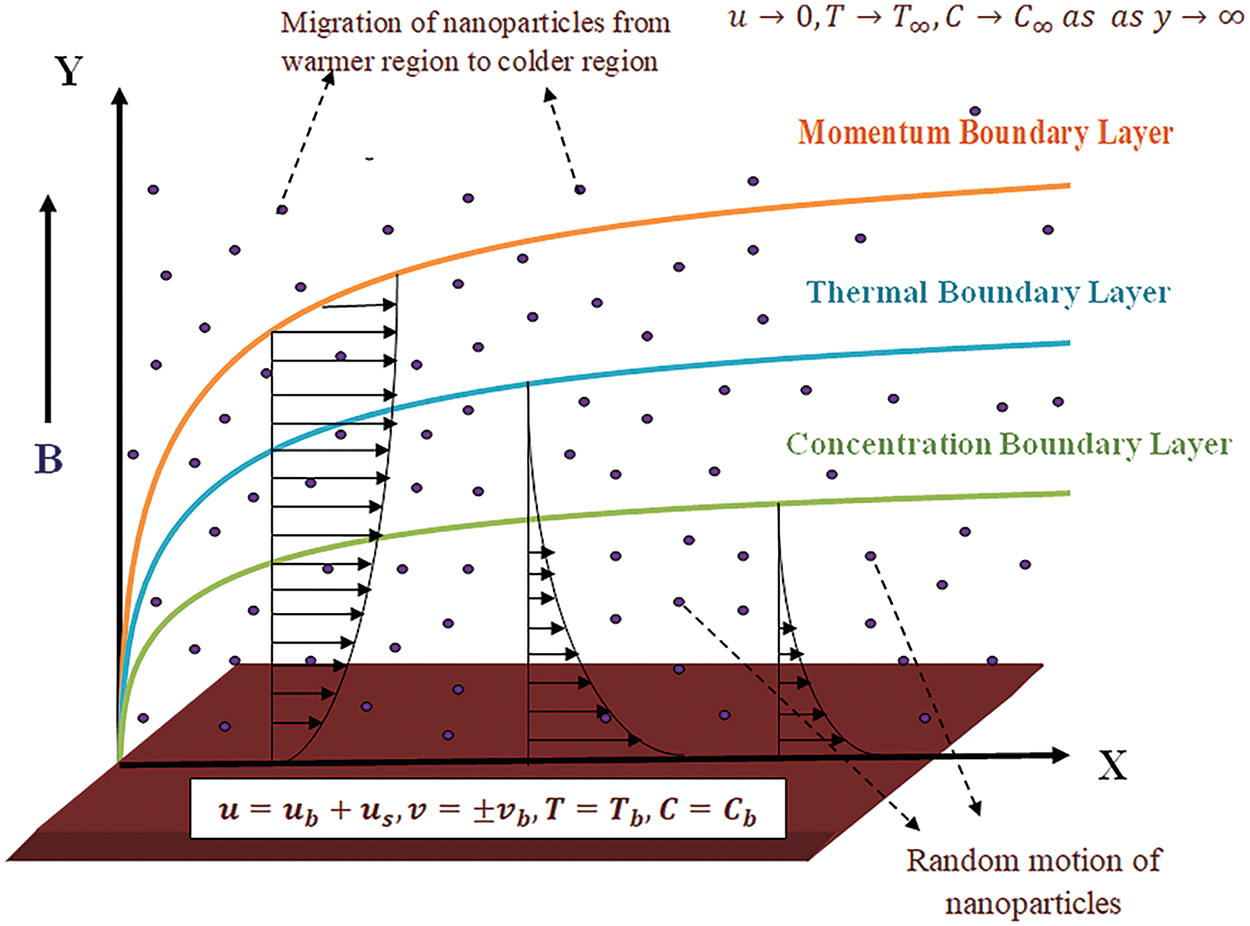
Figure 1: Physical geometry of the problem
A power-proportional relationship between velocity and the distance of a point from the slit on the plate is established to approximate the boundary layer flow of the nanofluid. The temperature at the stretching surface remains constant, as defined by
where
under boundary conditions
In this context, the vector
Here
Non-linear coupled differential equations are formulated based on the boundary layer Eqs. (5)–(7), yielding the following expressions:
and boundary conditions in (8) becomes
In this notation, derivatives with respect to
This parameter forces us to find local solution. Reconstructing
Here
Integrating the engineered physical parameters and the variables of interest for analysis—namely, the Nusselt number, Sherwood number, and skin friction—the designated parameters for the posed problem are delineated as follows:
where
These terms represent, respectively, the heat flux localized at a specific point, the mass flux concentrated in a particular area, and the wall shear stress exerted on the surface of the expanding sheet. The non-dimensional form of the skin friction, Nusselt number and Sherwood number are described as follows:
where
The analytical solution of the boundary value problem (BVP), as outlined in Eqs. (11)–(13), is not possible due to high nonlinearity. Therefore, the Eqs. (11)–(13) are first converted to a first-order system and then executed using the bvp4c module of the computational software MATLAB. The bvp4c module of MATLAB is a powerful tool for solving boundary value problems (BVPs) arising from ordinary differential equations (ODEs). This module is based on the collocation method, which is a well-established technique for solving BVPs. One of the major advantages of the bvp4c module is its ability to easily handle complex BVPs. It can also solve problems with multiple solutions, singularities, and discontinuities, making it a versatile tool for a wide range of applications. Additionally, the bvp4c solver is highly efficient and can solve large-scale problems quickly and accurately. Another significant advantage of bvp4c is its stability. The solver uses an adaptive mesh refinement strategy, which ensures that the solution is computed with high accuracy and stability. This is particularly important for problems that exhibit sensitive dependence on initial conditions or have multiple solutions. The bvp4c solver is also robust and can handle problems with stiff or oscillatory behavior. In light of its several advantages, bvp4c module of MATLAB is employed to find the solution of the equations governing the stated model. To enable solution using bvp4c, Eqs. (11)–(13) are transformed into a system of first-order differential equations using the suitably defined transformations.
Considering
the first-order system becomes
with boundary conditions
The system of first-order differential equations obtained in Eq. (20), along with boundary conditions in Eq. (21), is utilized within the bvp4c built-in routine, which implements the collocation technique in MATLAB, facilitating the acquisition of numerical results. Tolerance of numerical values is taken up to order
4 Numerical Results and Discussion
The current investigation embarks on the numerical resolution of differential Eqs. (11) to (13) while adhering to the boundary conditions (14). This endeavor employs collocation method inbuilt in bvp4c module of computational software MATLAB. The investigation encompasses scenarios involving both Constant Surface Temperature (CST) and Prescribed Surface Temperature (PST) for comparative analysis. Herein,
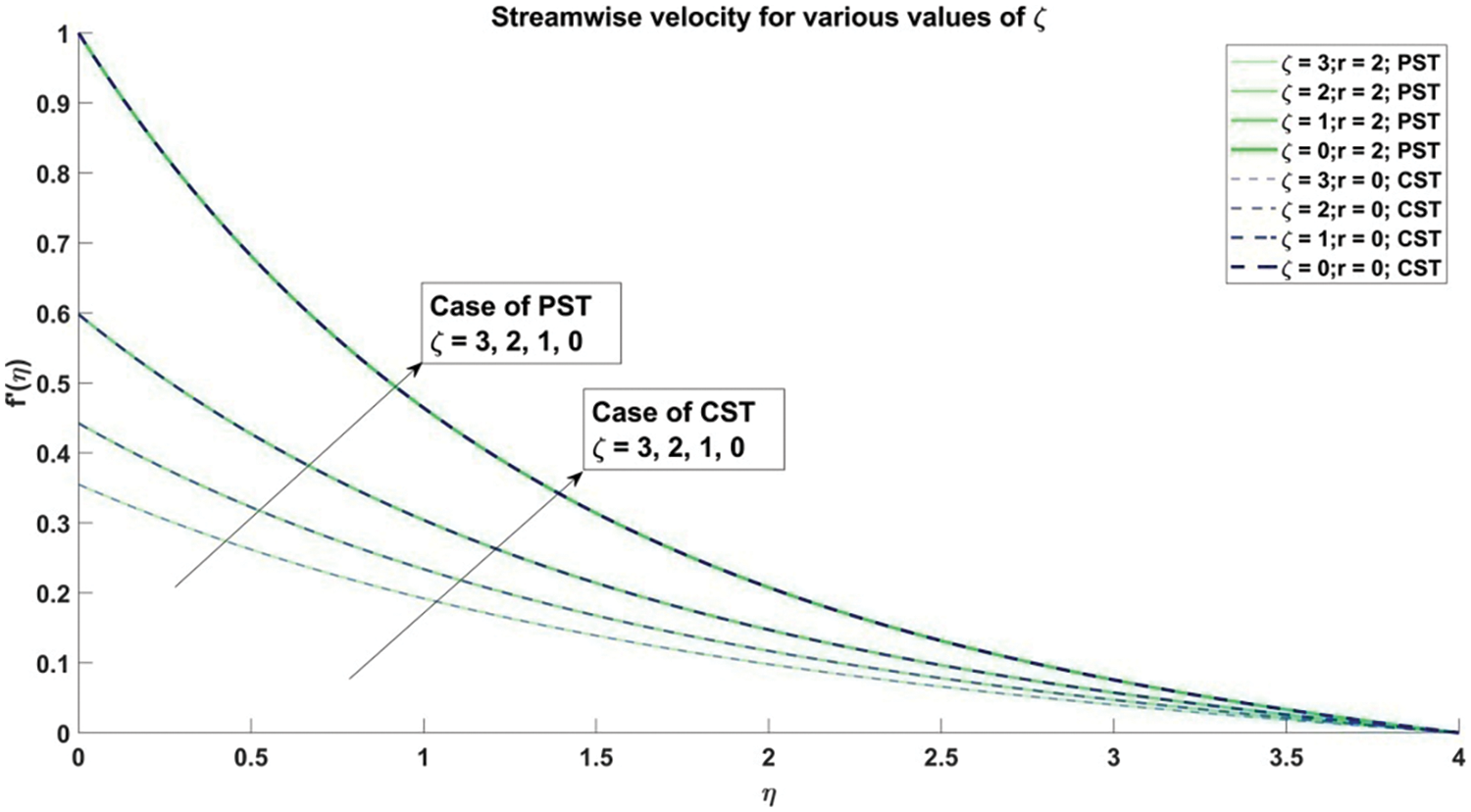
Figure 2: Stream-wise velocities corresponding to different values of
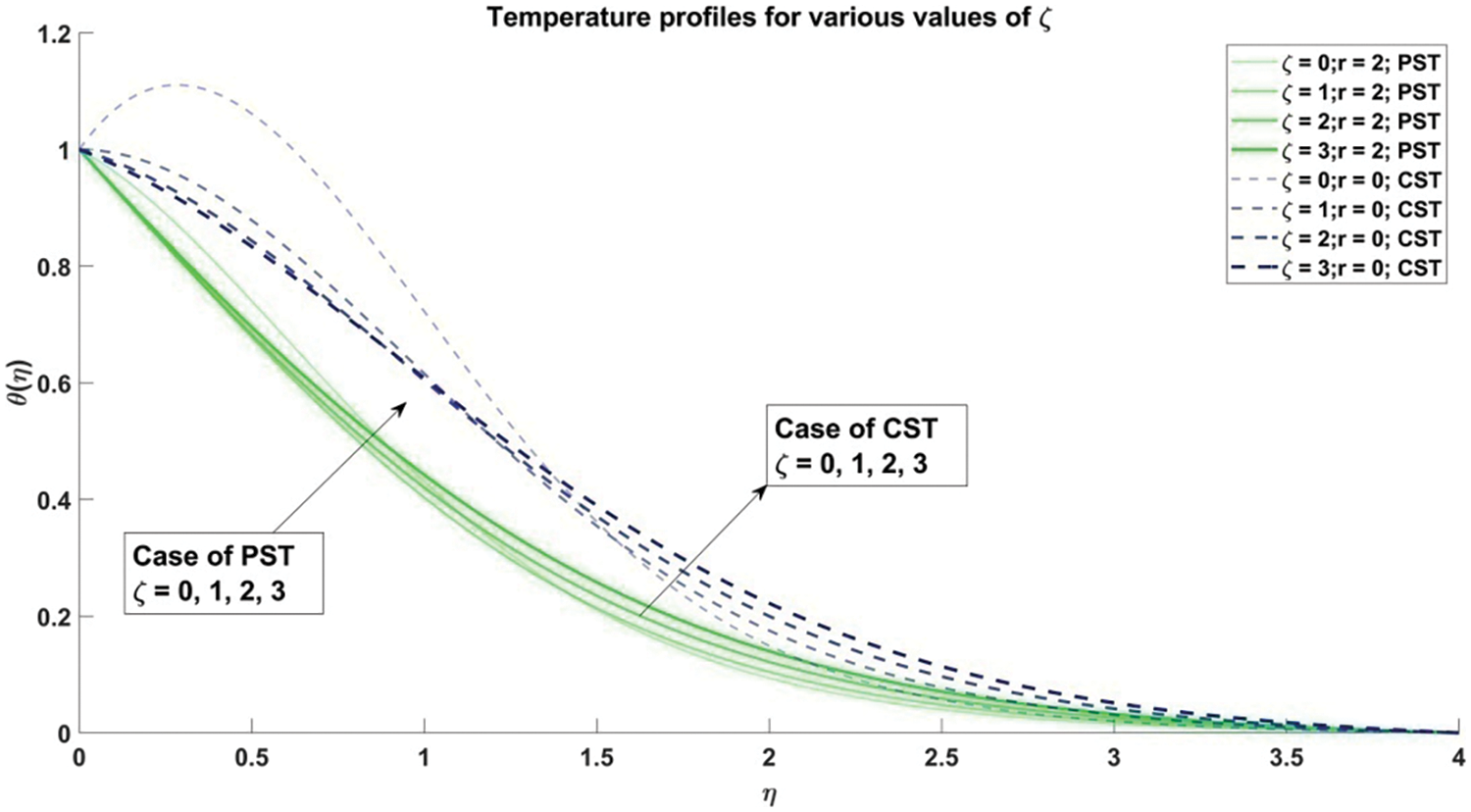
Figure 3: Temperature profiles corresponding to different values of slip parameter
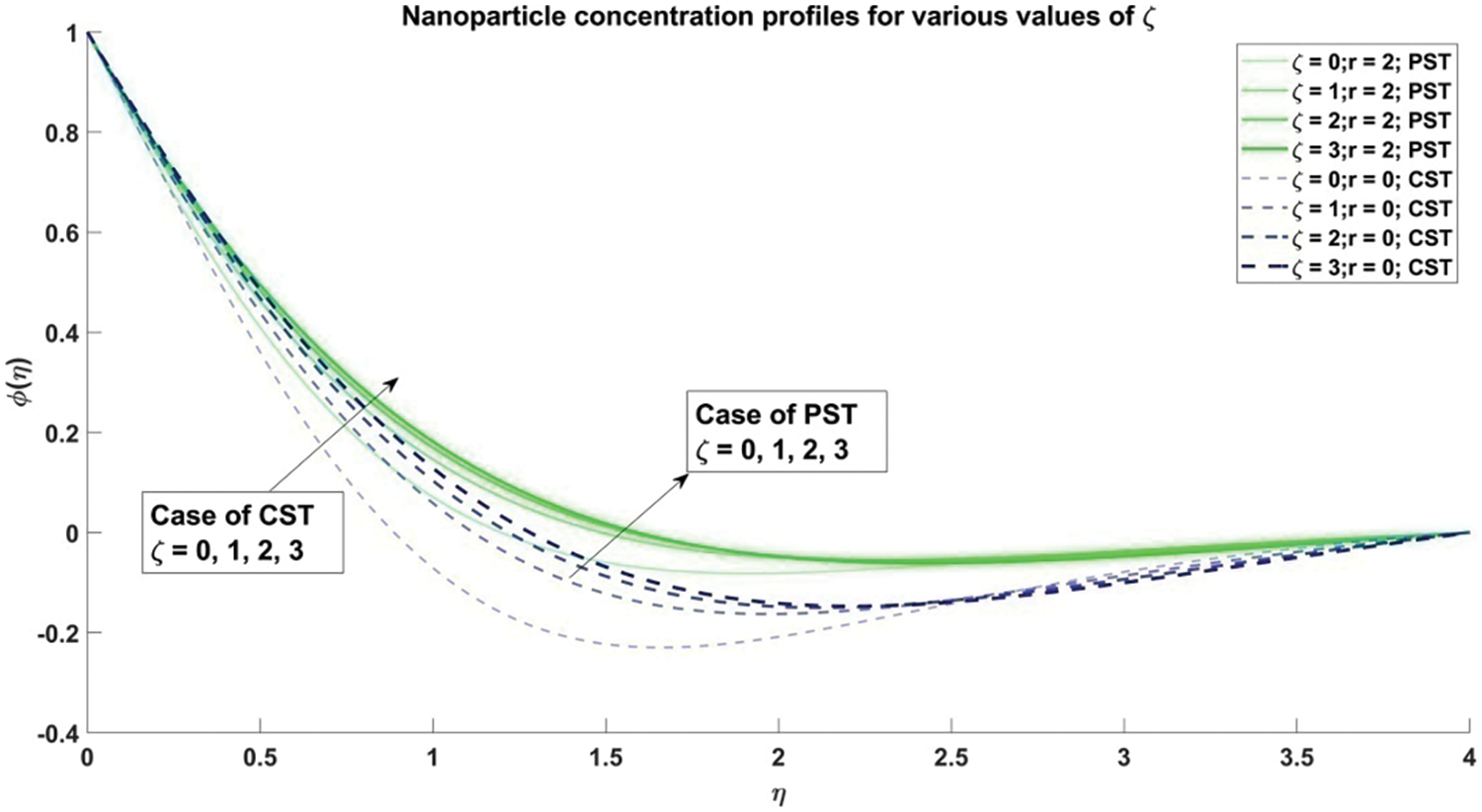
Figure 4: Concentration gradients of nanoparticles corresponding to various configurations of slip parameter
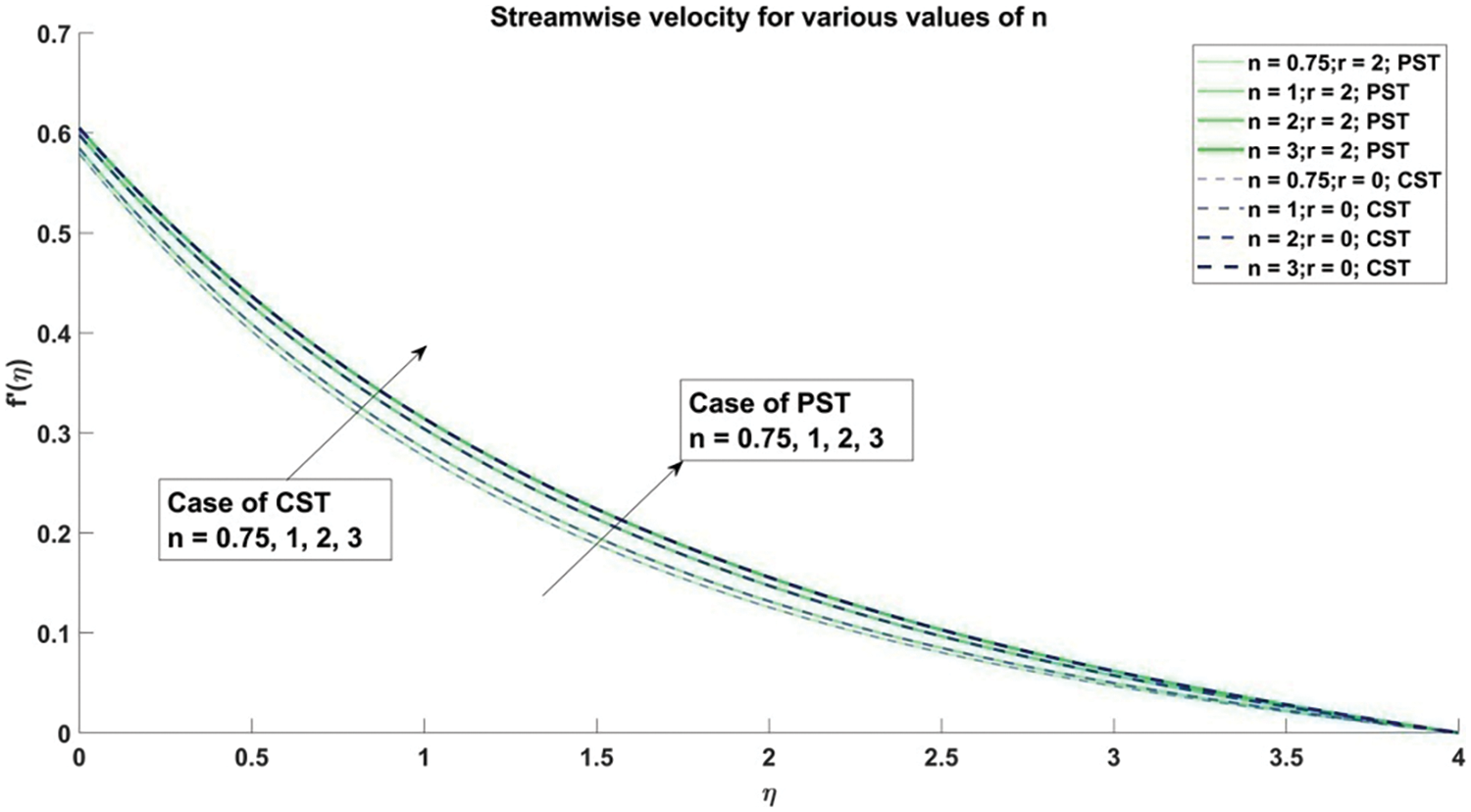
Figure 5: Stream-wise velocity corresponding to different values of n
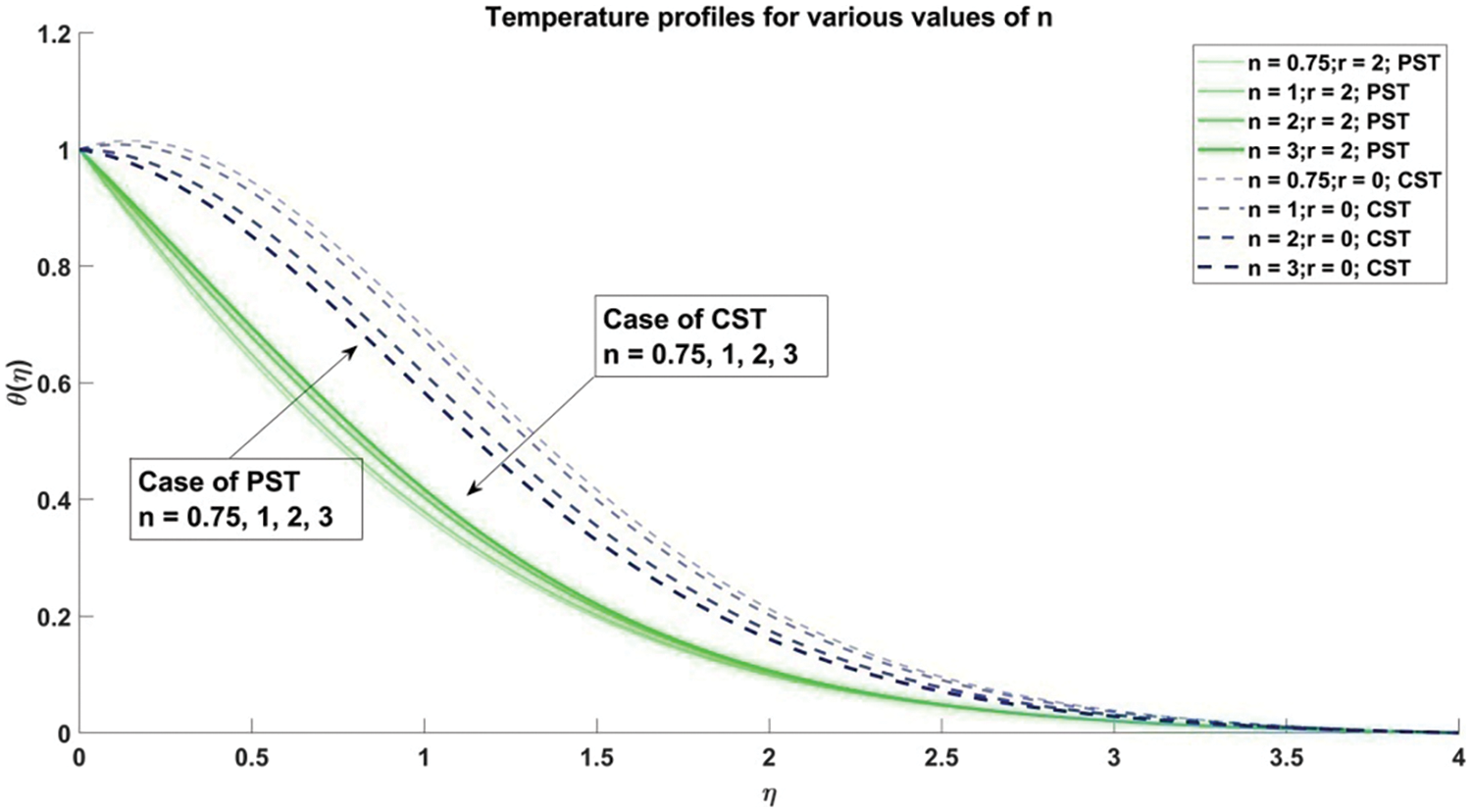
Figure 6: Profiles of temperature corresponding to different values of n
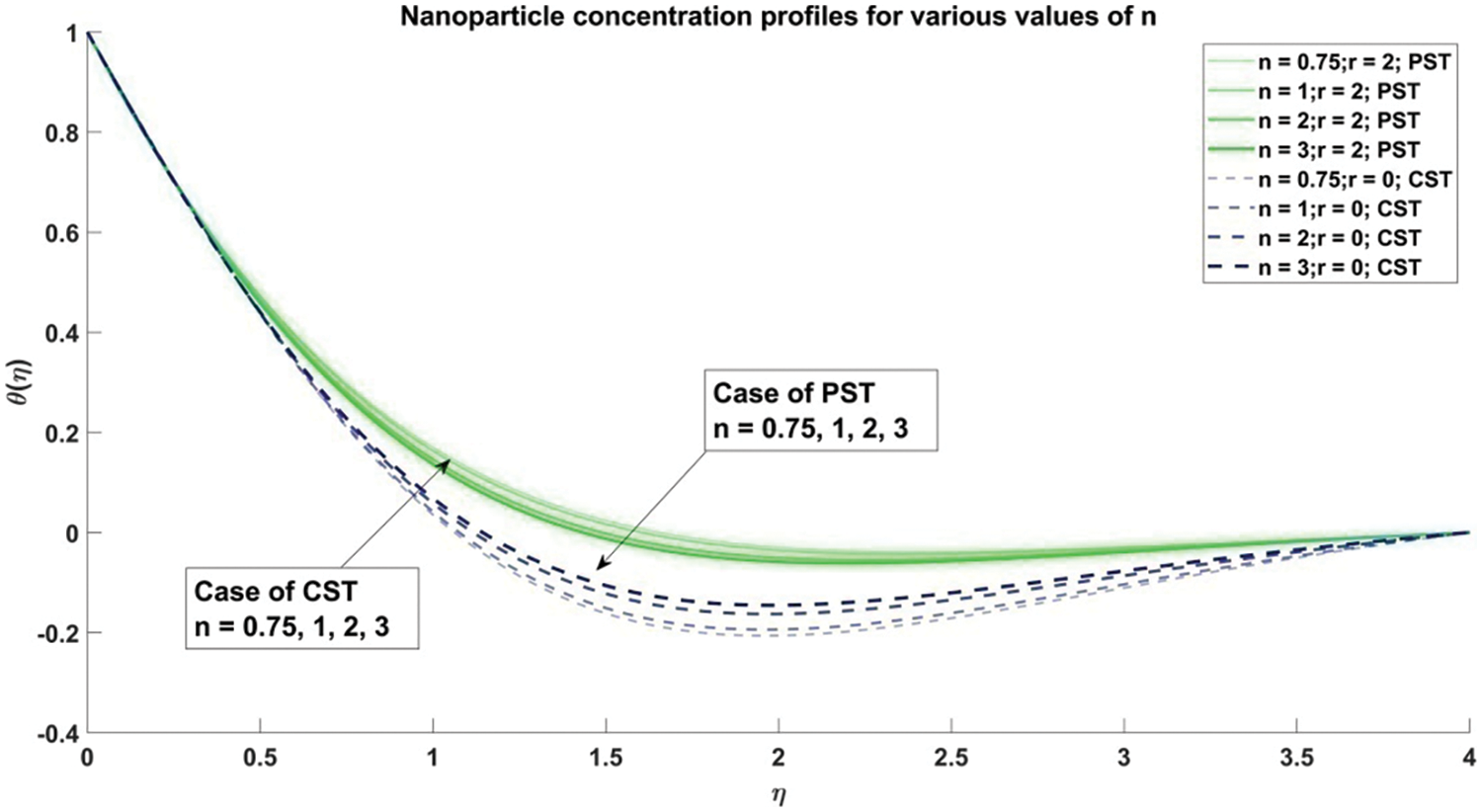
Figure 7: Profiles of concentration corresponding to different values of n
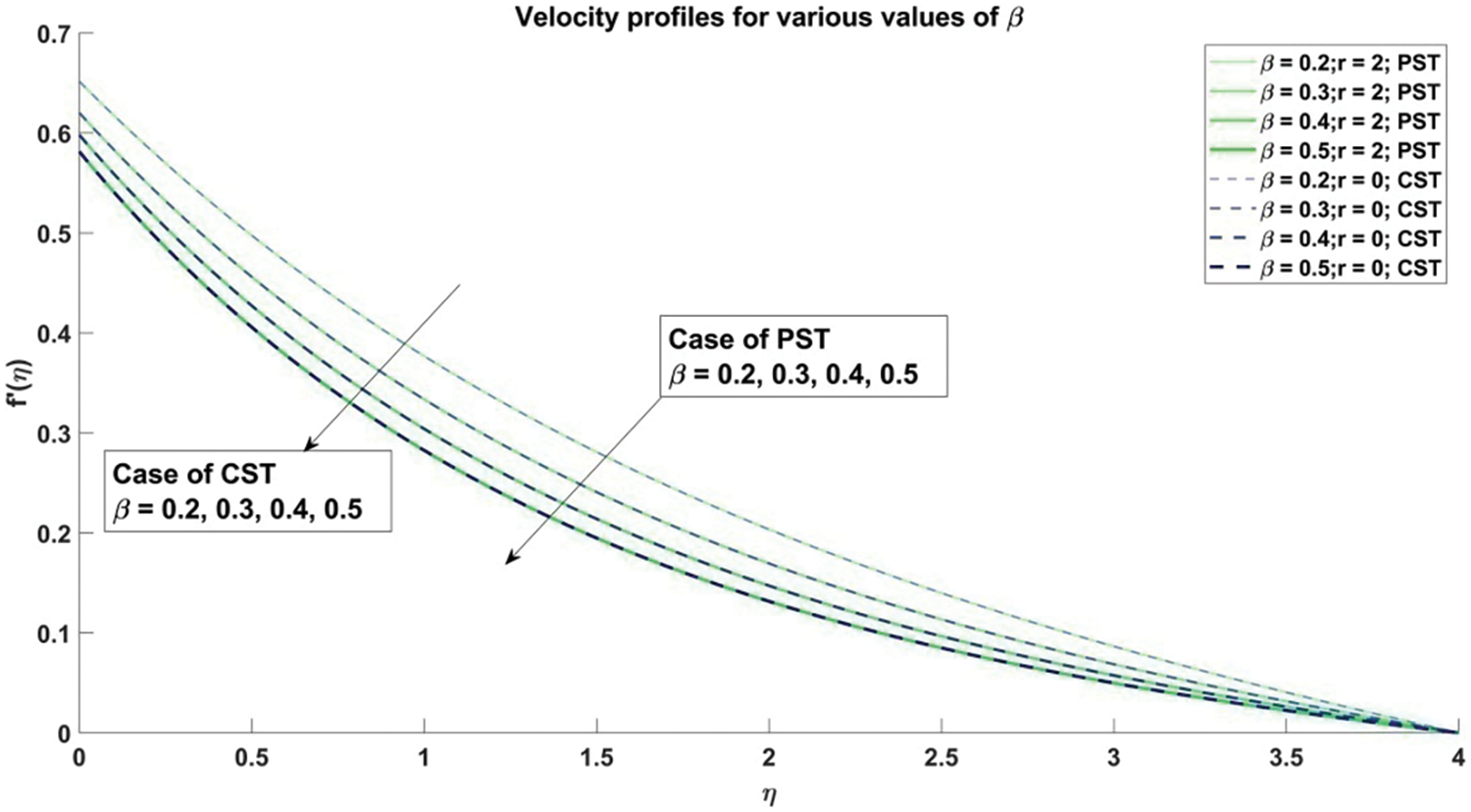
Figure 8: Profiles for velocity corresponding to different values of β
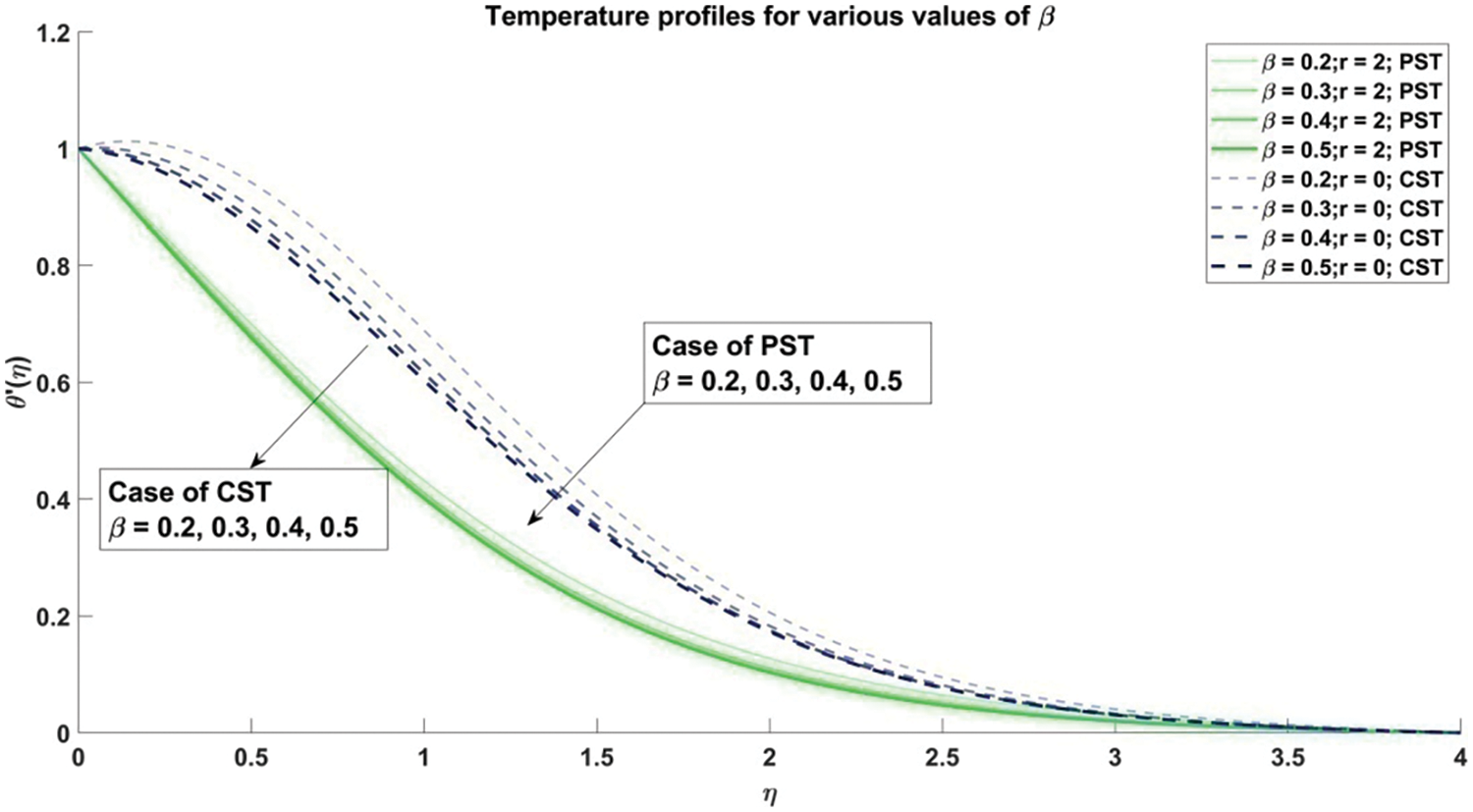
Figure 9: Profiles for temperature corresponding to different values of β
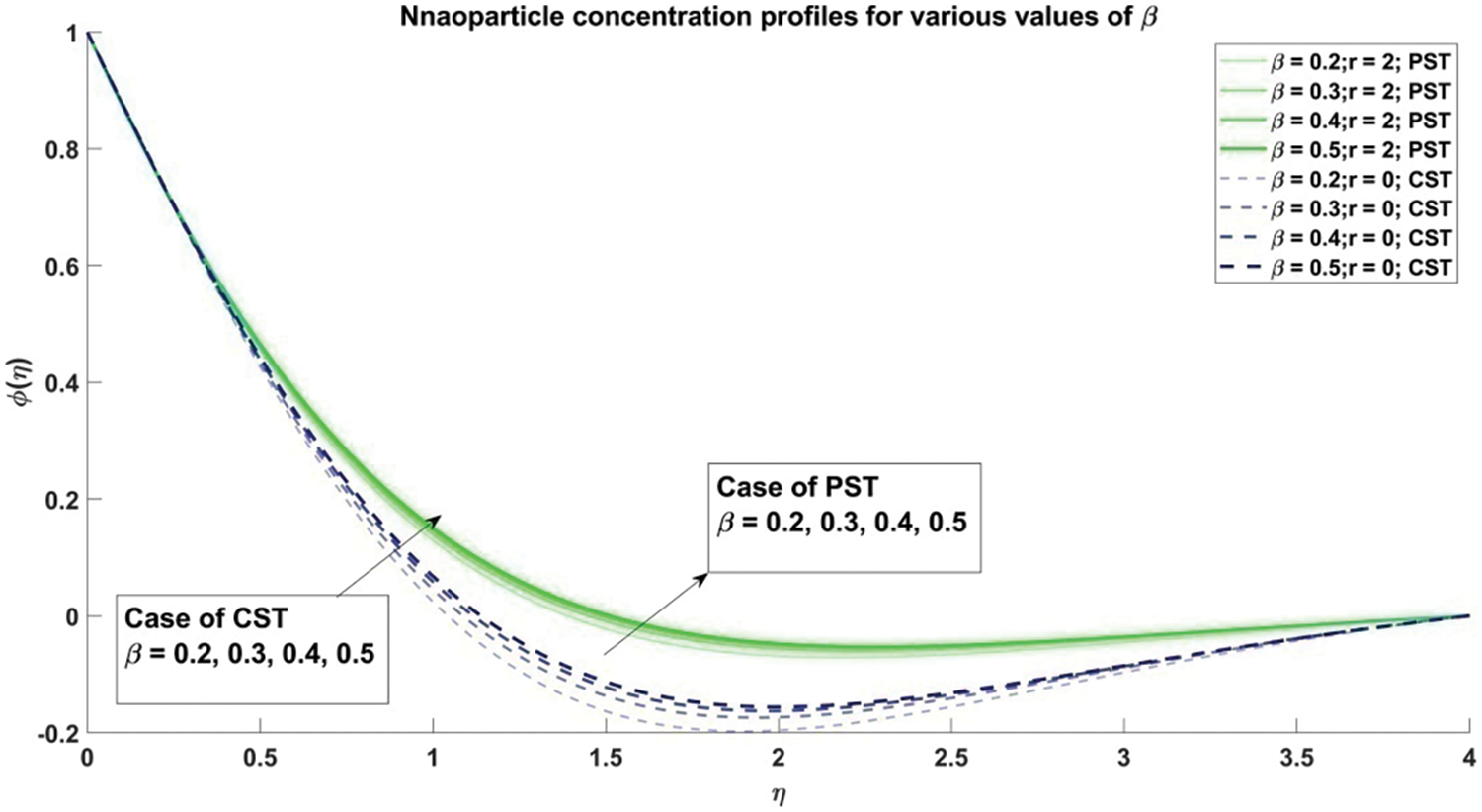
Figure 10: Profiles for nanoparticle concentration corresponding to different values of β
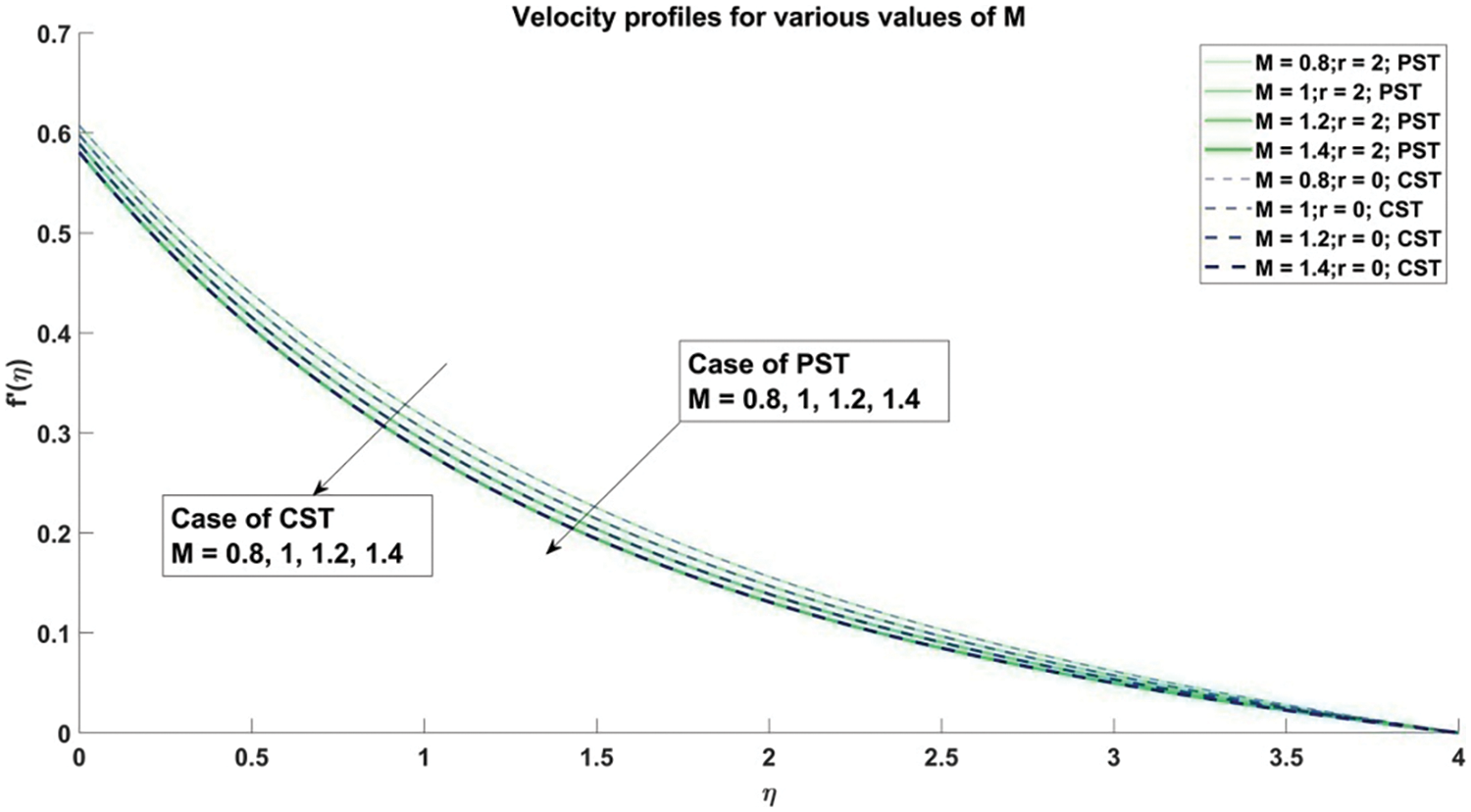
Figure 11: Profiles for velocity corresponding to different values of M
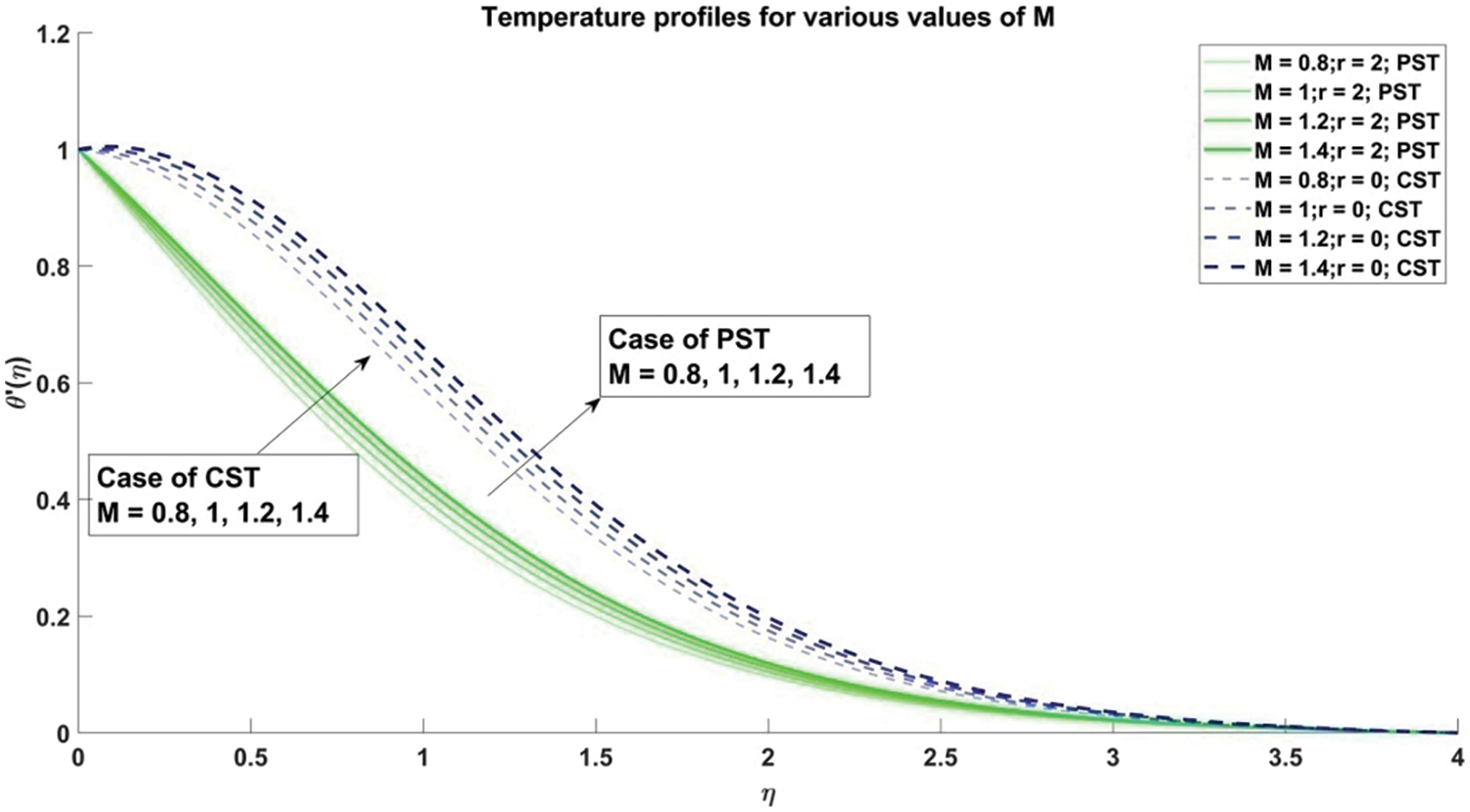
Figure 12: Profiles for temperature corresponding to different values of M
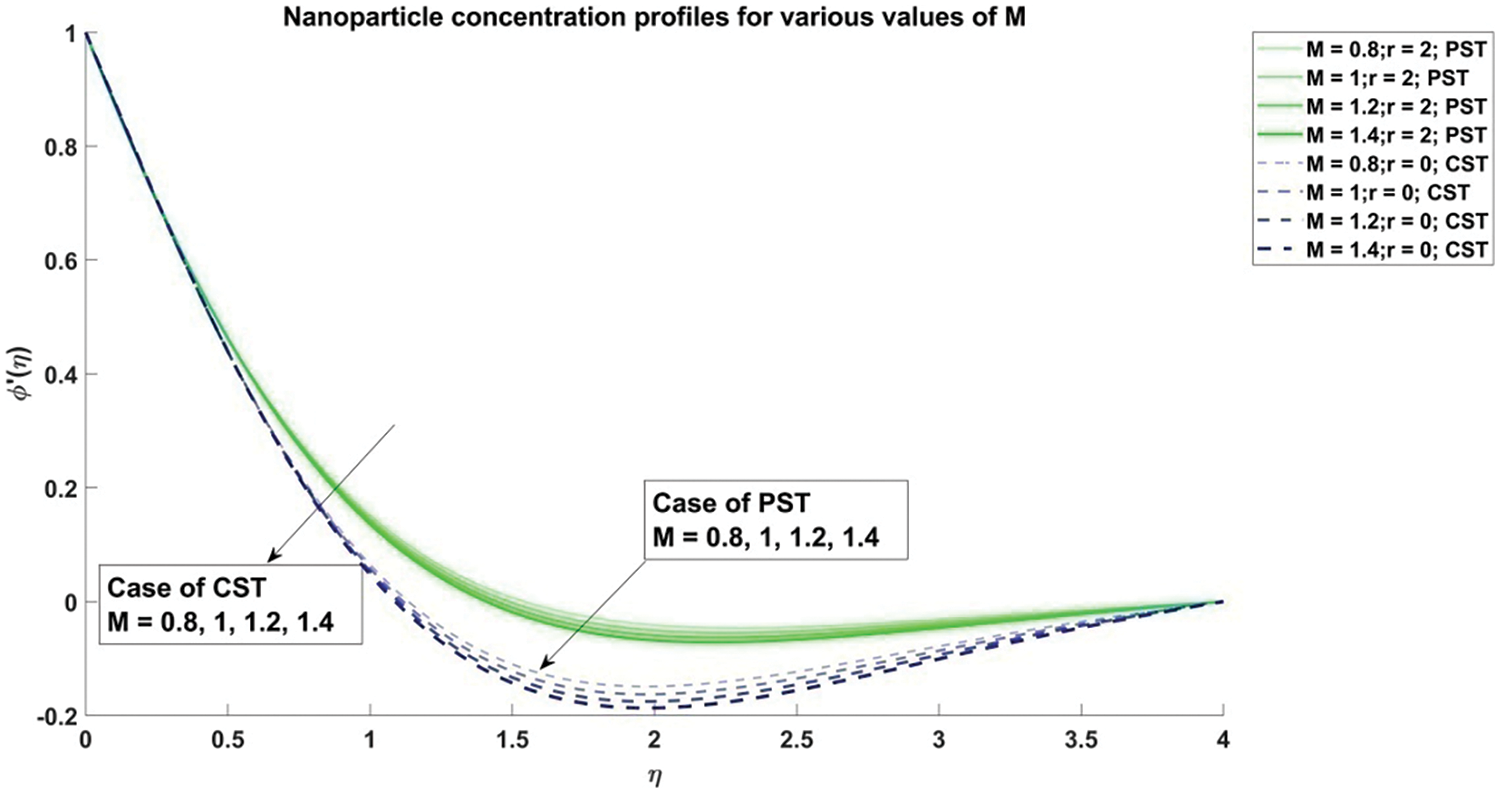
Figure 13: Profiles for nanoparticle concentration corresponding to different values of M
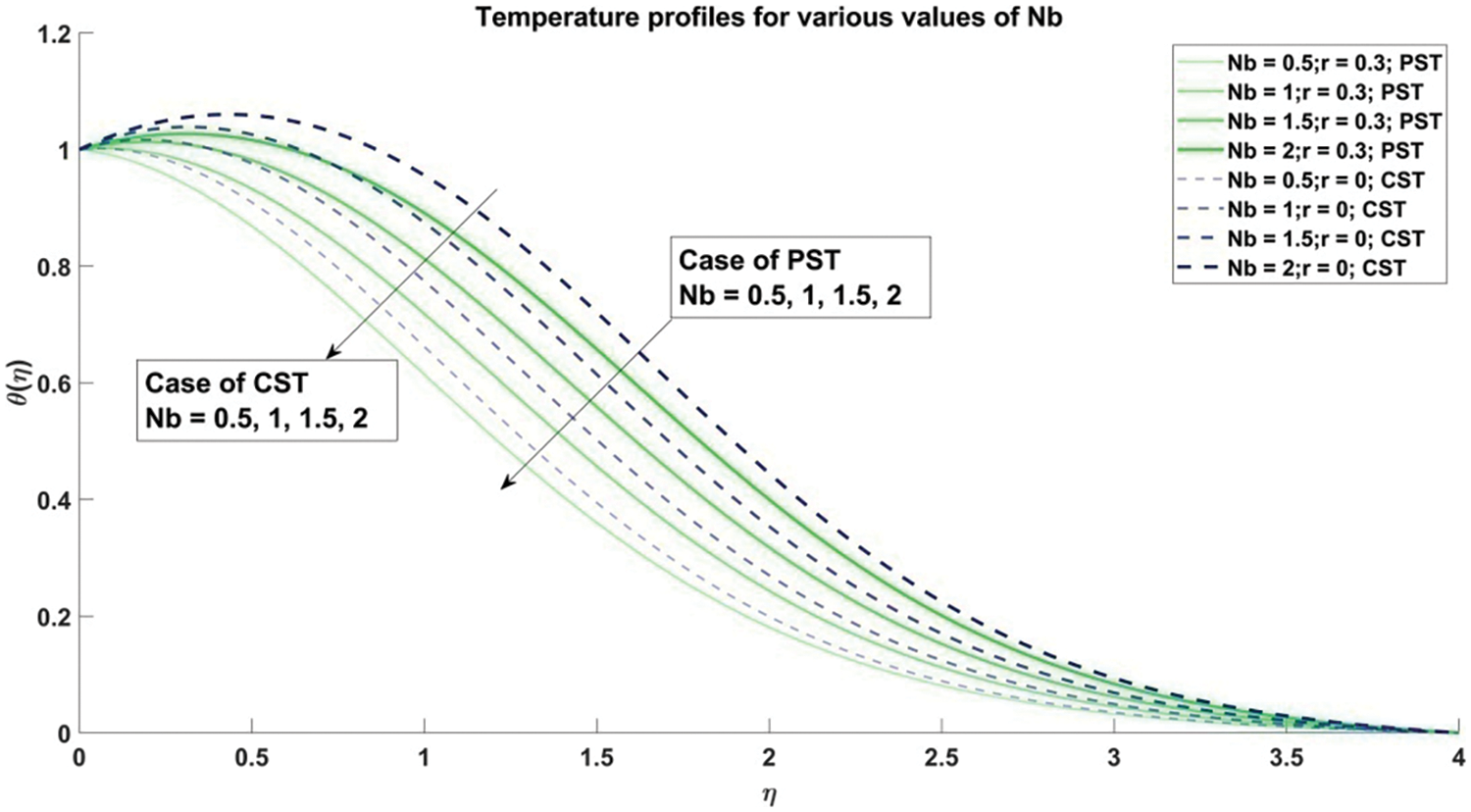
Figure 14: Profiles of temperature corresponding to different values of Nb
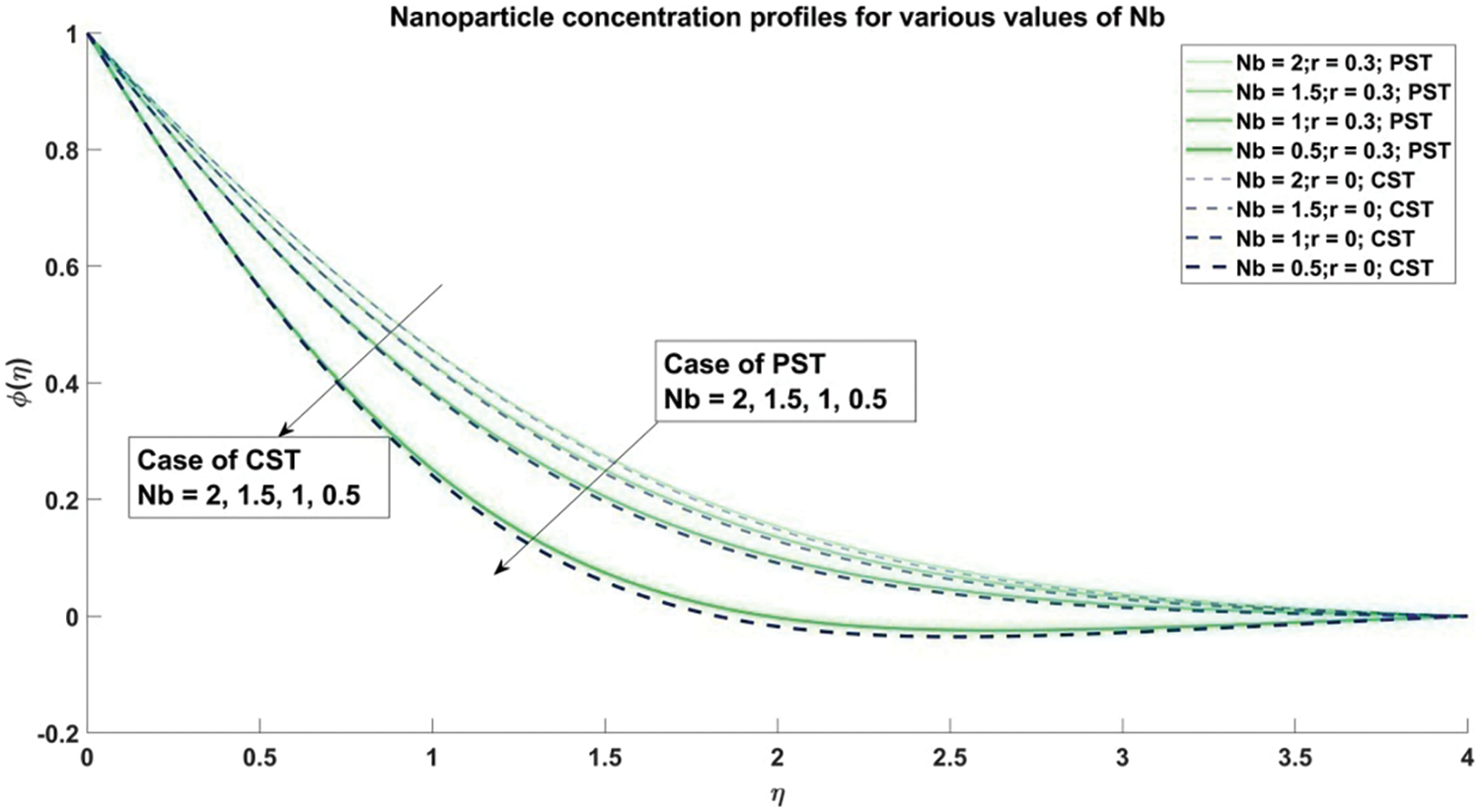
Figure 15: Nanoparticle concentration profiles corresponding to different values of Nb
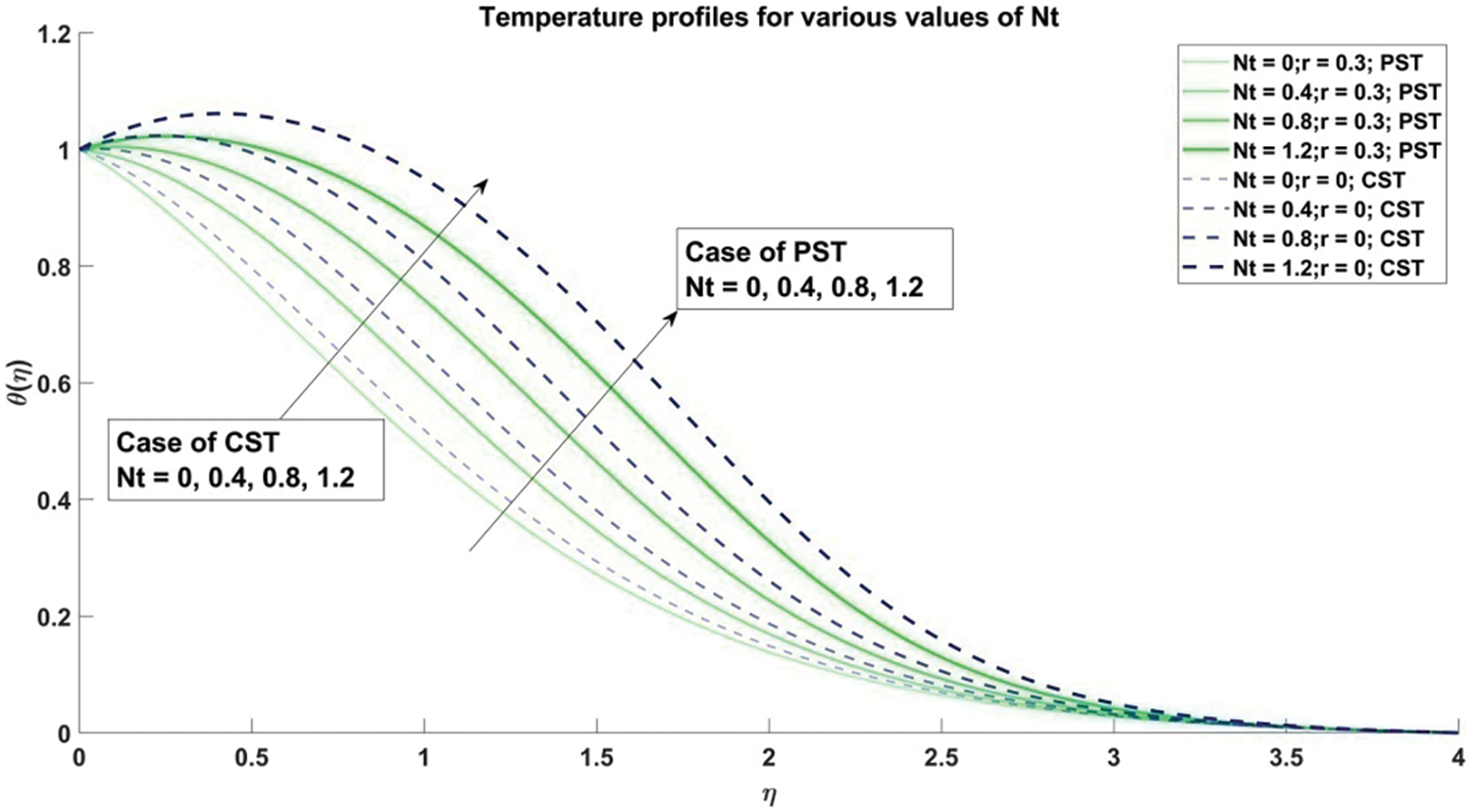
Figure 16: Profiles of temperature corresponding to different values of Nt
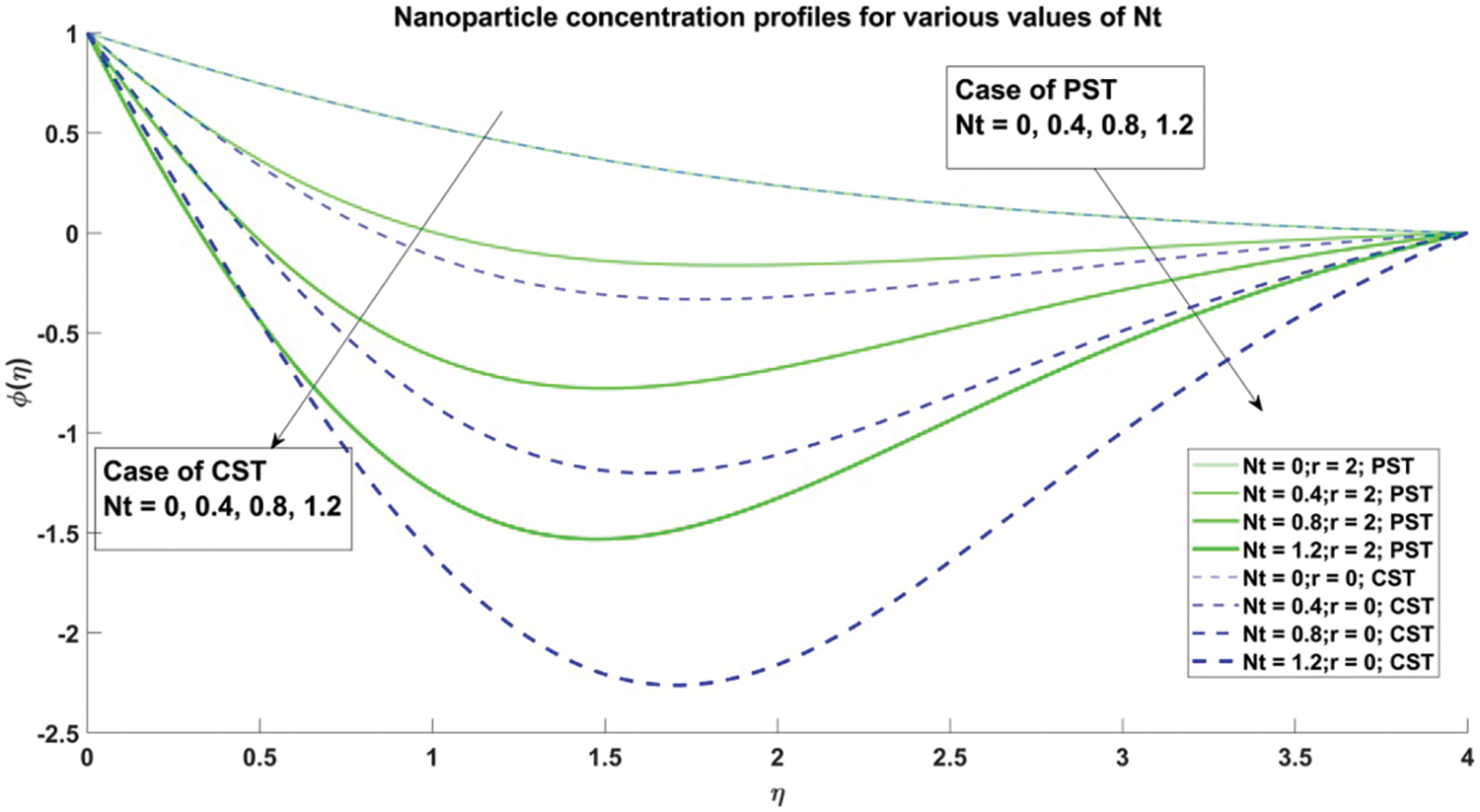
Figure 17: Profiles for nanoparticle concentration corresponding to different values of Nt
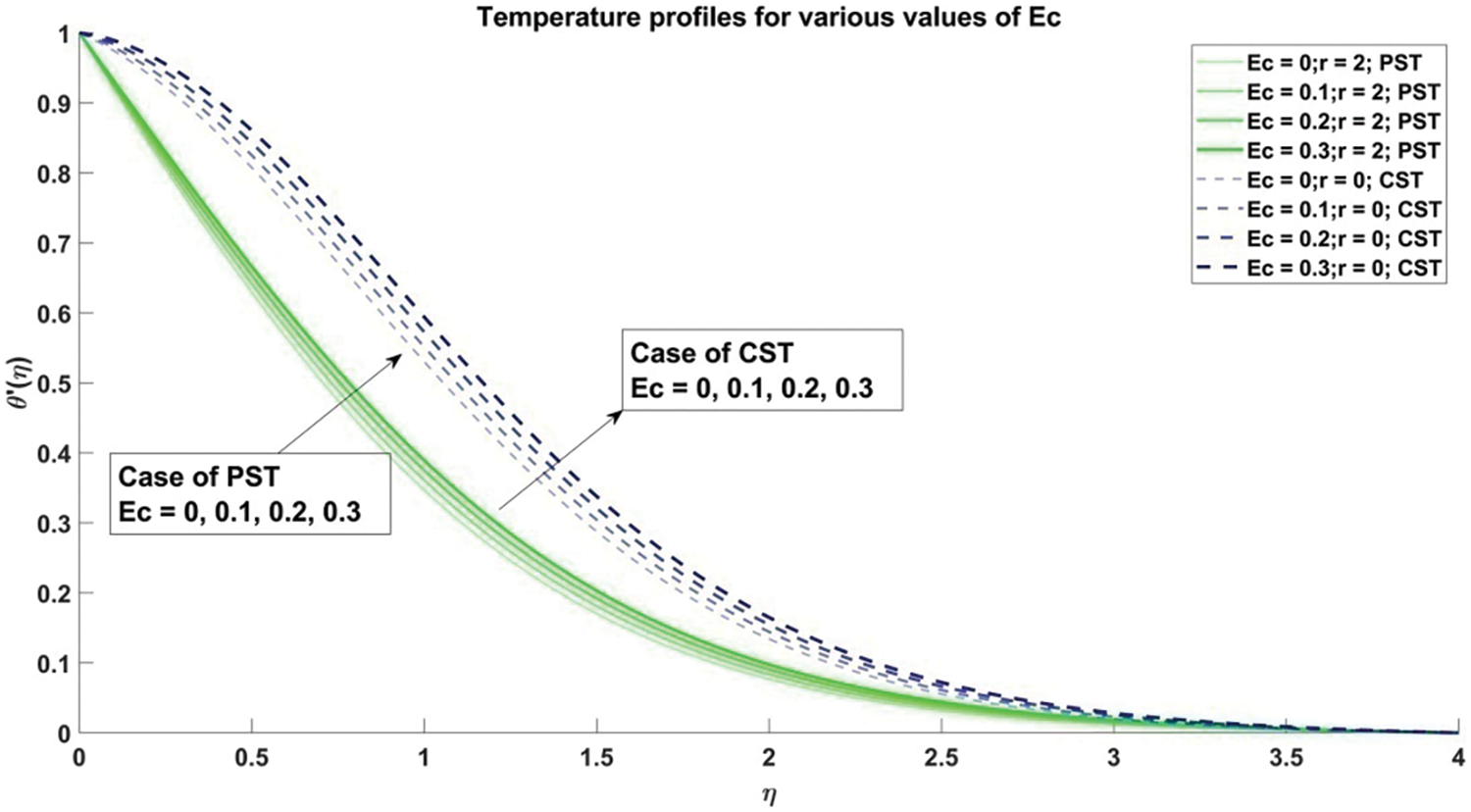
Figure 18: Profiles for temperature corresponding to different values of Ec
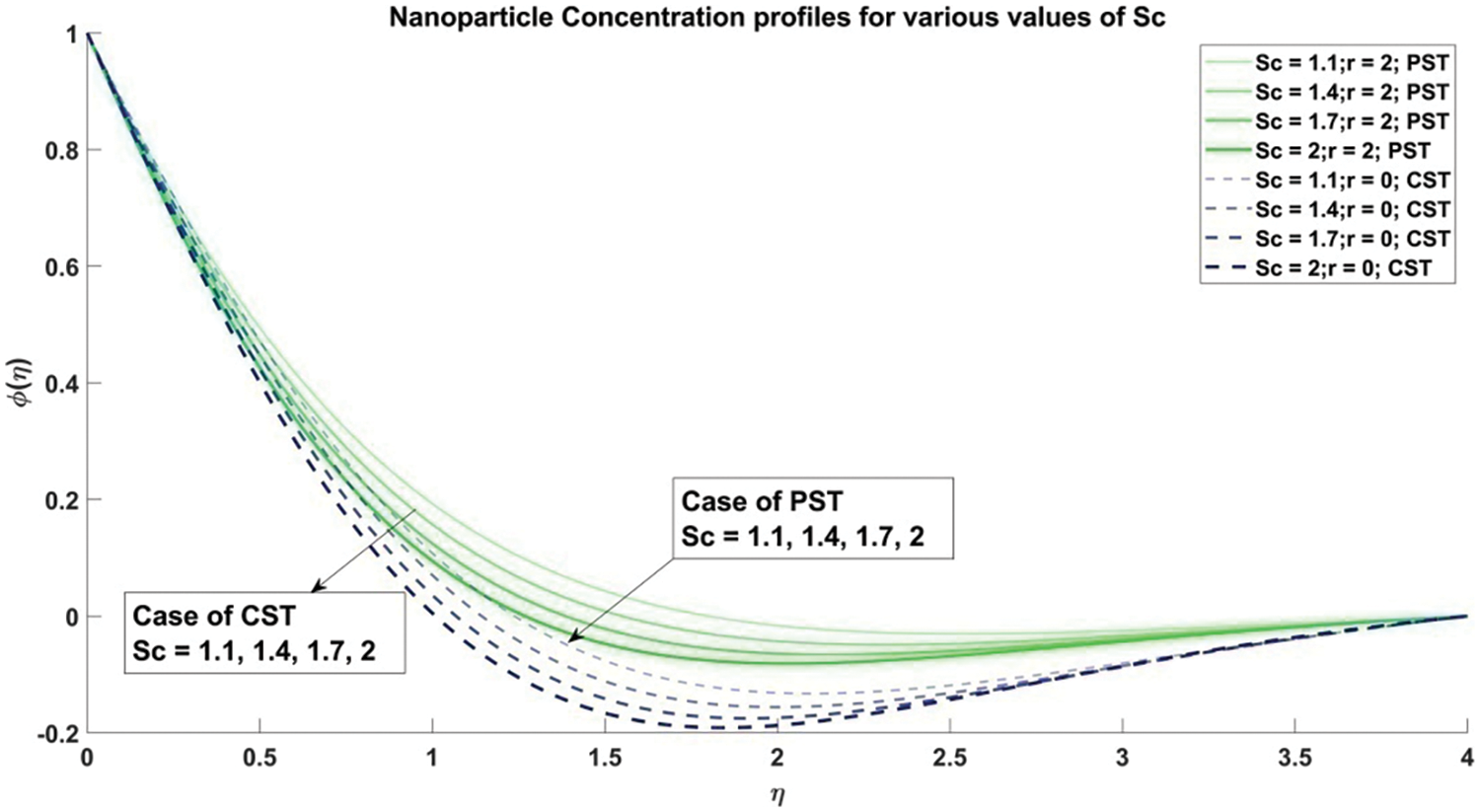
Figure 19: Profiles for nanoparticle concentration corresponding to different values of Sc
Fig. 2 presents a detailed exploration into the intricate ramifications of the slip parameter on the streamwise velocity characteristics of Casson nanofluid. Spanning across both Prescribed Surface Temperature (PST) and Constant Surface Temperature (CST) regimes, this graph meticulously examines the diverse magnitudes of the slip parameter, denoted by
Fig. 8 provides a comprehensive elucidation of the profound influence wielded by the Casson parameter
The scrutiny of the Casson fluid parameter
The decrement in velocity distribution is thus attributed to an escalation in the value of the magnetic parameter
Fig. 14 meticulously elucidates the intricate repercussions of Brownian motion parameter
In Fig. 15, a meticulous examination analyzes how the Brownian motion parameter
In Fig. 17, concentration profiles for various values of the thermophoresis parameter
Table 1 presents the validation of the current results by comparing the computed Nusselt number with values reported in existing studies, specifically for the parameters
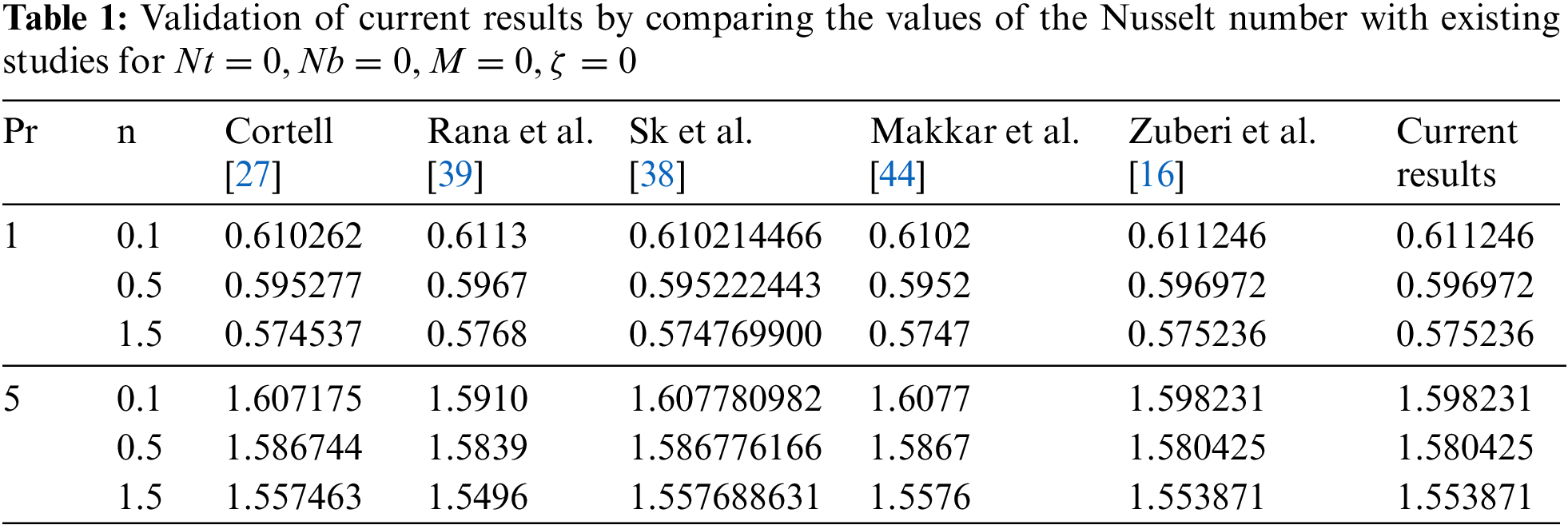
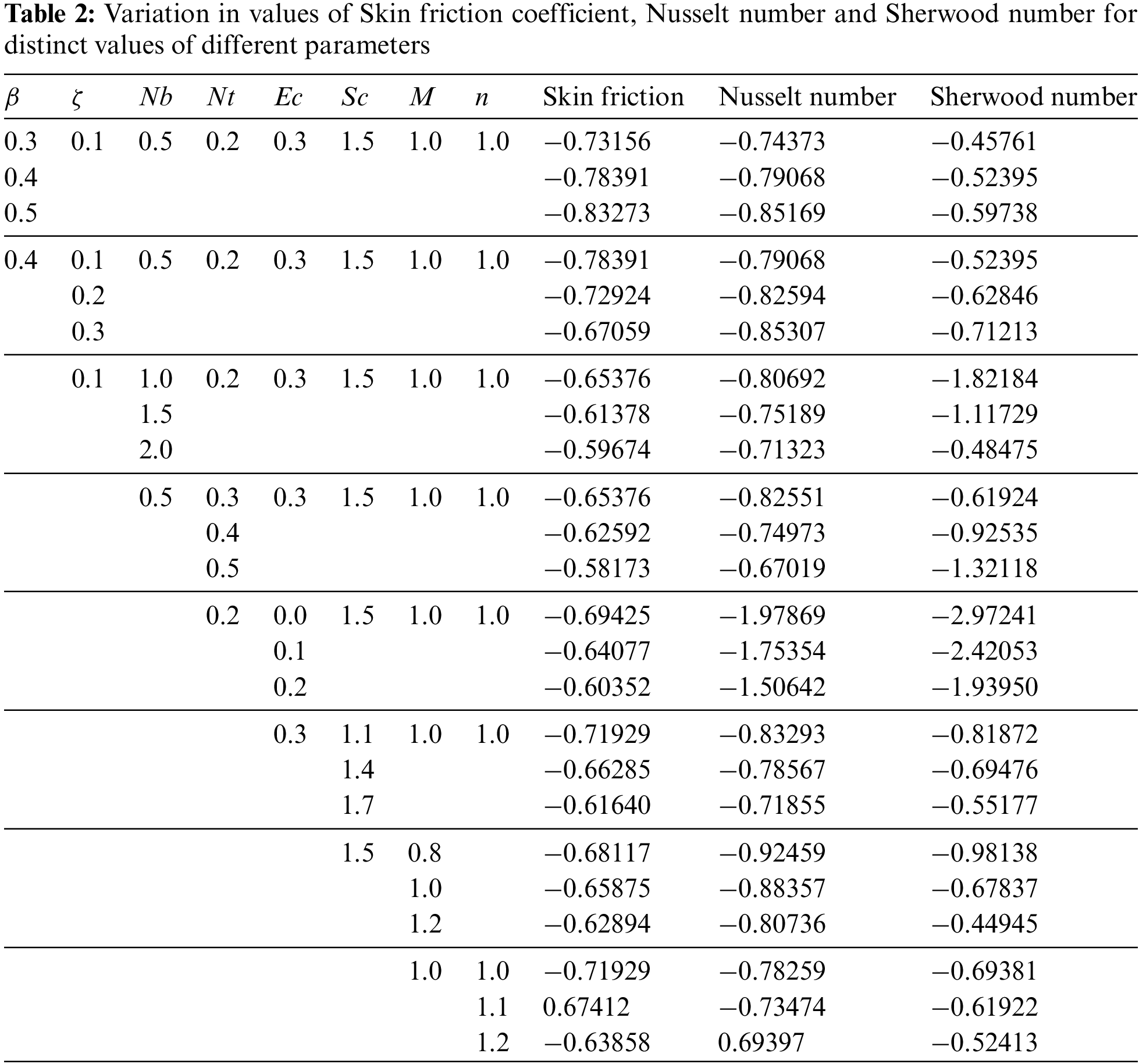
Table 2 delves into the variations in the values of the Skin Friction Coefficient, Nusselt number, and Sherwood number for distinct parameter settings, including
The present investigation delves into the intricate dynamics of mass, heat, and concentration transfer exhibited by a magnetohydrodynamic (MHD) Casson nanofluid interacting with a non-linearly stretched sheet. Emphasizing the inclusion of viscous and Ohmic dissipation effects, the study offers insights into the temperature distribution, volume fraction of nanoparticles, and the velocity profile of MHD Casson fluid flow. Moreover, the investigation meticulously explores both the scenarios of Constant Surface Temperature (CST) and Particular Surface Temperature (PST). Leveraging similarity conversion techniques, the governing partial differential equations are transformed into ordinary differential equations, necessitating a numerical exploration of the problem using the bvp4c module within MATLAB. Key conclusions drawn from the present analysis are summarized as follows:
1) As the magnetic parameter (
2) Observations reveal a decline in the velocity profile as the Casson fluid parameter (
3) The interplay of the parameter of slip and curved deformation parameter manifests in the thermal boundary layer thickness, where an escalation is observed for Prescribed Surface Temperature (PST), leading to an increase in temperature profiles. Conversely, for Constant Surface Temperature (CST), a reduction in the thermal boundary layer thickness ensues, consequently resulting in a decline in temperature profiles.
4) The concentration of nanoparticles exhibits a decline as the values of the Brownian motion parameter and thermophoresis parameter escalate, observed consistently across both Constant Surface Temperature (CST) and Prescribed Surface Temperature (PST) scenarios.
5) The temperature of the nanofluid demonstrates a notable increase corresponding to the escalation of both the Brownian motion parameter and thermophoresis parameter across both Constant Surface Temperature (CST) and Prescribed Surface Temperature (PST) conditions.
6) As the Schmidt number
7) The rise in the values of the viscous dissipation parameter (Eckert number
The study lacks the experimental validation. Also, the shape and size of nanoparticles have not been taken into account. Further, the results of the stated model are valid only for non-linear stretching sheet. Thus, the study can be explored further by taking into account the parameters of shape and size of nanoparticles. Also, the case of flow over shrinking sheet can be discussed, which will enhance our understanding of surface dynamics in various contexts. Moreover, the experimental validation by medical experts will benchmark the simulated results of the governing stated model.
Acknowledgement: The authors wish to extend their profound gratitude to Universiti Teknikal Malaysia Melaka (UTeM) for the unconditional support that enabled this research to be conducted. Also, the authors would like to express sincere thanks to all contributors whose hard work, knowledge, and teamwork were crucial to the accomplishment of this research.
Funding Statement: This research was funded by Universiti Teknikal Malaysia Melaka and Ministry of Higher Education (MoHE) Malaysia, grant number FRGS/1/2024/FTKM/F00586.
Author Contributions: The authors confirm their contribution to the paper as follows: Study conception and design: Haris Alam Zuberi, Madan Lal, Nurul Amira Zainal; Data collection: Haris Alam Zuberi, Madan Lal, Shivangi Verma, Nurul Amira Zainal; Analysis and interpretation of results: Haris Alam Zuberi, Madan Lal, Nurul Amira Zainal; Draft manuscript preparation: Haris Alam Zuberi, Madan Lal, Shivangi Verma, Nurul Amira Zainal. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Critical Care. 2016;20:1–13. [Google Scholar]
2. Caplan RM. Heart disease and hypertension. In: Long life strategy: a guide for living a longer, healthier, and more fulfilling life. Switzerland AG: Springer; 2024. p. 109–24. [Google Scholar]
3. Faghy MA, Tatler A, Chidley C, Fryer S, Stoner L, Laddu D, et al. The physiologic benefits of optimizing cardiorespiratory fitness and physical activity–from the cell to systems level in a post-pandemic world. Prog Cardiovasc Dis. 2024;33:49–54. [Google Scholar]
4. Srivastava L, Srivastava V. Peristaltic transport of blood: Casson model—II. J Biomech. 1984;17(11):821–9. [Google Scholar] [PubMed]
5. Ali F, Sheikh NA, Khan I, Saqib M. Magnetic field effect on blood flow of Casson fluid in axisymmetric cylindrical tube: a fractional model. J Magn Magn Mater. 2017;423:327–36. [Google Scholar]
6. Pandey R, Kumar M, Majdoubi J, Rahimi-Gorji M, Srivastav VK. A review study on blood in human coronary artery: numerical approach. Comput Methods Programs Biomed. 2020;187:105243. [Google Scholar] [PubMed]
7. Mauter MS, Zucker I, Perreault F, Werber JR, Kim JH, Elimelech M. The role of nanotechnology in tackling global water challenges. Nat Sustain. 2018;1(4):166–75. doi:10.1038/s41893-018-0046-8. [Google Scholar] [CrossRef]
8. Wiek A, Foley RW, Guston DH. Nanotechnology for sustainability: what does nanotechnology offer to address complex sustainability problems?. In: Nanotechnology for sustainable development. Switzerland AG: Springer; 2014. p. 371–90. [Google Scholar]
9. Saxer T, Holme MN. Challenges in cardiovascular treatments using nanotechnology-based approaches. Nanosci Nanotechnol Human Health. 2016;51–70. [Google Scholar]
10. Qi C, Luo T, Liu M, Fan F, Yan Y. Experimental study on the flow and heat transfer characteristics of nanofluids in double-tube heat exchangers based on thermal efficiency assessment. Energy Convers Manag. 2019;197(39):111877. doi:10.1016/j.enconman.2019.111877. [Google Scholar] [CrossRef]
11. Kim J, Lee N, Hyeon T. Recent development of nanoparticles for molecular imaging. Philos Trans Royal Society A: Math, Phys Eng Sci. 2017;375(2107):20170022. doi:10.1098/rsta.2017.0022. [Google Scholar] [PubMed] [CrossRef]
12. Bhaumik B, Changdar S, De S. Combined impact of Brownian motion and thermophoresis on nanoparticle distribution in peristaltic Nanofluid flow in an asymmetric channel. Int J Ambient Energy. 2022;43(1):5064–75. doi:10.1080/01430750.2021.1934539. [Google Scholar] [CrossRef]
13. Mörters P, Peres Y. Brownian motion. Cambridge, UK: Cambridge University Press; 2010, vol. 30. [Google Scholar]
14. Piazza R, Parola A. Thermophoresis in colloidal suspensions. J Phys: Condens Matter. 2008;20(15):153102. doi:10.1088/0953-8984/20/15/153102. [Google Scholar] [CrossRef]
15. Dubey A, Vasu B, Anwar Bég O, Gorla RS, Kadir A. Computational fluid dynamic simulation of two-fluid non-Newtonian nanohemodynamics through a diseased artery with a stenosis and aneurysm. Comput Methods Biomech Biomed Eng. 2020;23(8):345–71. doi:10.1080/10255842.2020.1729755. [Google Scholar] [PubMed] [CrossRef]
16. Zuberi HA, Lal M, Verma S, Zainal NA. Computational investigation of brownian motion and thermophoresis effect on blood-based casson nanofluid on a non-linearly stretching sheet. J Adv Res Numer Heat Transfer. 2024;18(1):49–67. doi:10.37934/arnht.18.1.4967. [Google Scholar] [CrossRef]
17. Madhura KR, Babitha. Numerical study on magnetohydrodynamics micropolar Carreau nanofluid with Brownian motion and thermophoresis effect. Int J Model Simul. 2023 Jul;16:1–4. doi:10.1080/02286203.2023.2234240. [Google Scholar] [CrossRef]
18. Reddy SBV, Ashwathnarayana DP, Mysore J, Chandrashekhar DV. Ohmic and viscous dissipation effect on free and forced convective flow of casson fluid in a channel. Biointerface Res Appl Chem. 2021;12(1):132–48. doi:10.33263/BRIAC. [Google Scholar] [CrossRef]
19. Tang TQ, Rooman M, Vrinceanu N, Shah Z, Alshehri A. Blood flow of Au-nanofluid using Sisko model in stenotic artery with porous walls and viscous dissipation effect. Micromachines. 2022;13(8):1303. doi:10.3390/mi13081303. [Google Scholar] [PubMed] [CrossRef]
20. Das S, Pal T, Jana R. Electromagnetic hybrid nano-blood pumping via peristalsis through an endoscope having blood clotting in presence of Hall and ion slip currents. BioNanoScience. 2021;11(3):848–70. doi:10.1007/s12668-021-00853-2. [Google Scholar] [CrossRef]
21. Yusuf TA. Analysis of entropy generation in nonlinear convection flow of unsteady magneto-nanofluid configured by vertical stretching sheet with Ohmic heating. Int J Ambient Energy. 2023 Dec 31;44(1):2319–35. doi:10.1080/01430750.2023.2236103. [Google Scholar] [CrossRef]
22. Siddiqui AA, Turkyilmazoglu M. Film flow of nano-micropolar fluid with dissipation effect. Comput Model Eng Sci. 2024 Jul 8;140(3):2487–512. doi:10.32604/cmes.2024.050525. [Google Scholar] [CrossRef]
23. Hsu HJ, Bugno J, Lee Sr, Hong S. Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip Rev: Nanomed Nanobiotechnology. 2017;9(1):e1409. doi:10.1002/wnan.1409. [Google Scholar] [PubMed] [CrossRef]
24. Kumar R. Lipid-based nanoparticles for drug-delivery systems. In: Nanocarriers for drug delivery. Amsterdam, Netherland: Elsevier; 2019. p. 249–84. [Google Scholar]
25. Nazar R, Amin N, Filip D, Pop I. Stagnation point flow of a micropolar fluid towards a stretching sheet. Int J Non-Linear Mech. 2004;39(7):1227–35. [Google Scholar]
26. Crane LJ. Flow past a stretching plate. Zeitschrift für angewandte Mathematik und Physik ZAMP. 1970;21:645–7. [Google Scholar]
27. Cortell R. Viscous flow and heat transfer over a nonlinearly stretching sheet. Appl Math Comput. 2007;184(2):864–73. [Google Scholar]
28. Alahmadi RA, Raza J, Mushtaq T, Abdelmohsen SA, Gorji R, Hassan M, et al. Optimization of MHD flow of radiative micropolar nanofluid in a channel by RSM: sensitivity analysis. Mathematics. 2023;11(4):939. [Google Scholar]
29. Raza J, Al-Khaled K, Dero S, Lund LA, Khan SU, Khan MI, et al. Heat and mass transfer phenomenon for micropolar nanofluid with microrotation effects: nonsimilarity simulations. Int J Mod Phys B. 2023;37(19):2350183. [Google Scholar]
30. Pandey AK, Kumar M. Natural convection and thermal radiation influence on nanofluid flow over a stretching cylinder in a porous medium with viscous dissipation. Alexandria Eng J. 2017;56(1):55–62. [Google Scholar]
31. Boujelbene M, Rehman S, Alqahtani S, Eldin SM. Optimizing thermal characteristics and entropy degradation with the role of Nanofluid flow configuration through an inclined channel. Alexandria Eng J. 2023;69:85–107. [Google Scholar]
32. Reddy BP, Shamshuddin MD, Salawu SO, Sademaki LJ. Computational analysis of transient thermal diffusion and propagation of chemically reactive magneto-nanofluid, Brinkman-type flow past an oscillating absorbent plate. Partial Differ Equ Appl Math. 2024 Jun 14;11:100761. [Google Scholar]
33. Thiriet M. Biology and mechanics of blood flows: part II: mechanics and medical aspects. New York, USA: Springer Science & Business Media; 2007. [Google Scholar]
34. Wang Y, Li H, Guo Y, Lee WN. Bidirectional ultrasound elastographic imaging framework for non-invasive assessment of the non-linear behavior of a physiologically pressurized artery. Ultrasoun Med Biol. 2019;45(5):1184–96. [Google Scholar]
35. Wajihah SA, Sankar D. A review on non-Newtonian fluid models for multi-layered blood rheology in constricted arteries. Arch Appl Mech. 2023;93(5):1771–96. doi:10.1007/s00419-023-02368-6. [Google Scholar] [PubMed] [CrossRef]
36. Saeed A, Khan N, Gul T, Kumam W, Alghamdi W, Kumam P. The flow of blood-based hybrid nanofluids with couple stresses by the convergent and divergent channel for the applications of drug delivery. Molecules. 2021;26(21):6330. doi:10.3390/molecules26216330. [Google Scholar] [PubMed] [CrossRef]
37. Yazdi MH, Abdullah S, Hashim I, Sopian K. Slip MHD liquid flow and heat transfer over non-linear permeable stretching surface with chemical reaction. Int J Heat Mass Transf. 2011 Jul 1;54(15–16):3214–25. doi:10.1016/j.ijheatmasstransfer.2011.04.009. [Google Scholar] [CrossRef]
38. Sk MT, Das K, Kundu PK. Effect of magnetic field on slip flow of nanofluid induced by a non-linear permeable stretching surface. Appl Therm Eng. 2016 Jul 5;104(3):758–66. doi:10.1016/j.applthermaleng.2016.05.129. [Google Scholar] [CrossRef]
39. Rana P, Bhargava R. Flow and heat transfer of a nanofluid over a nonlinearly stretching sheet: a numerical study. Commun Nonlinear Sci Numer Simul. 2012 Jan 1;17(1):212–26. doi:10.1016/j.cnsns.2011.05.009. [Google Scholar] [CrossRef]
40. Qayyum M, Riaz MB, Afzal S. Analysis of blood flow of unsteady Carreau-Yasuda nanofluid with viscous dissipation and chemical reaction under variable magnetic field. Heliyon. 2023 Jun 1;9(6):e16522. [Google Scholar]
41. Chaudhary S, Singh A, Kumar D, Baleanu D. Numerical analysis for MHD blood-nanofluid flow through a non-linearly stretched sheet interpolated in a permeable medium along heat generation. Case Stud Therm Eng. 2023 Dec 1;52:103786. [Google Scholar]
42. Zari I, Gul T, Dosmagulova K, Khan TS, Haq S. Heat transfer analysis of radiative-marangoni convective flow in nanofluid comprising Lorentz forces and porosity effects. Adv Theory Nonlinear Anal its Appl. 2023;7(1):61–81. [Google Scholar]
43. Hou E, Nazir U, Naz S, Sohail M, Nadeem M, Lee JR, et al. Novel analysis of two kinds hybrid models in ferro martial inserting variable Lorentz force past a heated disk: an implementation of finite element method. Comput Model Eng Sci. 2023;135(2):1393–411. doi:10.32604/cmes.2022.022500. [Google Scholar] [CrossRef]
44. Makkar V, Poply V, Goyal R, Sharma N. Numerical investigation of mhd casson nanofluid flow towards a non linear stretching sheet in presence of double-diffusive effects along with viscous and ohmic dissipation. J Therma Eng. 2021;7(2):1–17. [Google Scholar]
45. Tawade JV, Guled C, Noeiaghdam S, Fernandez-Gamiz U, Govindan V, Balamuralitharan S. Effects of thermophoresis and Brownian motion for thermal and chemically reacting Casson nanofluid flow over a linearly stretching sheet. Results Eng. 2022;15(3):100448. doi:10.1016/j.rineng.2022.100448. [Google Scholar] [CrossRef]
46. Hussain S, Rasheed K, Ali A, Vrinceanu N, Alshehri A, Shah Z. A sensitivity analysis of MHD nanofluid flow across an exponentially stretched surface with non-uniform heat flux by response surface methodology. Sci Rep. 2022;12(1):18523. doi:10.1038/s41598-022-22970-y. [Google Scholar] [PubMed] [CrossRef]
47. Gao S, Yang X, Xu J, Qiu N, Zhai G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano. 2021;15(8):12567–603. doi:10.1021/acsnano.1c02103. [Google Scholar] [PubMed] [CrossRef]
48. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24. doi:10.1038/s41573-020-0090-8. [Google Scholar] [PubMed] [CrossRef]
49. Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng. 2013;15(1):253–82. doi:10.1146/annurev-bioeng-071812-152409. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools