 Open Access
Open Access
ARTICLE
Fuzzy Difference Equations in Diagnoses of Glaucoma from Retinal Images Using Deep Learning
1 Department of Mathematics, Voorhees College, Vellore, Tamil Nadu, India
2 Chandigarh Group of Colleges, Jhanjeri, Mohali, Punjab, India
3 Department of Computer Engineering, College of Computer and Information Sciences, King Saud University, P.O. Box 51178, Riyadh, 11543, Saudi Arabia
4 Faculty of Science and Technology, School of IT and Systems, University of Canberra, Canberra, Australia
5 Operations Research Department, Faculty of Graduate Studies for Statistical Research, Cairo University, Giza, 12613, Egypt
6 Applied Science Research Center, Applied Science Private University, Amman, 11937, Jordan
* Corresponding Authors: Ali Wagdy Mohamed. Email: ,
# Lord Buddha Education Foundation, Kathmandu, Nepal (When the paper was submitted, Dr. Kautish was in LBEF Campus, Nepal. But currently he is in Chandigarh Group of Colleges, Jhanjeri, Mohali, Punjab, India.)
(This article belongs to the Special Issue: Advances in Ambient Intelligence and Social Computing under uncertainty and indeterminacy: From Theory to Applications)
Computer Modeling in Engineering & Sciences 2024, 139(1), 801-816. https://doi.org/10.32604/cmes.2023.030902
Received 01 May 2023; Accepted 21 September 2023; Issue published 30 December 2023
Abstract
The intuitive fuzzy set has found important application in decision-making and machine learning. To enrich and utilize the intuitive fuzzy set, this study designed and developed a deep neural network-based glaucoma eye detection using fuzzy difference equations in the domain where the retinal images converge. Retinal image detections are categorized as normal eye recognition, suspected glaucomatous eye recognition, and glaucomatous eye recognition. Fuzzy degrees associated with weighted values are calculated to determine the level of concentration between the fuzzy partition and the retinal images. The proposed model was used to diagnose glaucoma using retinal images and involved utilizing the Convolutional Neural Network (CNN) and deep learning to identify the fuzzy weighted regularization between images. This methodology was used to clarify the input images and make them adequate for the process of glaucoma detection. The objective of this study was to propose a novel approach to the early diagnosis of glaucoma using the Fuzzy Expert System (FES) and Fuzzy differential equation (FDE). The intensities of the different regions in the images and their respective peak levels were determined. Once the peak regions were identified, the recurrence relationships among those peaks were then measured. Image partitioning was done due to varying degrees of similar and dissimilar concentrations in the image. Similar and dissimilar concentration levels and spatial frequency generated a threshold image from the combined fuzzy matrix and FDE. This distinguished between a normal and abnormal eye condition, thus detecting patients with glaucomatous eyes.Keywords
Glaucoma occurs due to damage to the nerve that connects the eye to the brain, known as the optic nerve. Glaucoma is one of the silent disorders that human beings can get without previous physical signs or symptoms. Tjandrasa et al. [1] assumed that individuals with glaucoma could lose their optic nerve tissues which could lead to losing their vision. Unfortunately, there is no cure for a glaucomatous eye. With that being said, early discovery of the glaucomatous eye can slow down the damage to the optic nerve which can fortunately save the affected individual’s vision. The Cup to Disk Ratio (CDR) is the primary tool used to check whether the individual is at hazard for glaucoma or not. The CDR and ISNT (inferior (I) > superior (S) > nasal (N) > temporal (T)) are the two key diagnostic tools used to evaluate the Optic Nerve Head (ONH). The CDR is a rule that was developed through manual estimation by an ophthalmologist. Despite its benefits, manually monitoring ONH adjustment is a time-consuming process. Since the early diagnosis of this disease is necessary to prevent vision loss, numerous efforts have been made to the programmed discovery of glaucoma at an early stage. Apart from this, optimized medical-aided diagnosis and treatment were analyzed by Zhang et al. [2].
This paper is structured as follows Section 2 provides a literature review of what has been previously done in this field of research. Section 3 discusses the concepts of intuitive fuzzy sets. Section 4 outlines the approach used in retinal image enhancement using fuzzy concepts and the implementation through CNN. This section also analyzes fuzzy manipulation and defuzzification techniques related to CDR. Section 5 deals with fuzzy image segmentation which is used to integrate all the detected regions and partition the image based on similar and dissimilar concentration levels. Spatial frequency fuzzy metric concepts are also discussed for generating a threshold image. In Section 6, numerical illustrations and the various results that were obtained are analyzed.
Haubecker et al. [3] proposed a neuro-fuzzy inference system to detect glaucoma using higher-order spectra (HOS) and discrete wavelet transform (DWT) which was based on the criterion of diffuse expulsion. The proposed system was tested on a series of actual ophthalmic images of normal and glaucomatous cases. The most important parameters were then extracted, after having recognized the elliptical form of both the optic disc and boundary. After extracting the highlights, a factual analysis was performed on the partitioned separation. Finally, Dehghani et al. [4] proposed an assortment of algorithmic rules based on fuzzy logic approaches to determine the condition of the patient’s eye. The condition of the eye was then distinguished into three categories including normal, mild glaucoma, and direct/severe glaucoma. Naik et al. suggested a fuzzy set theory-based image enhancement method on HSV (hue, saturation, value) images by applying Gaussian distribution on S and V parameters [5].
The optic disc cup and the parameters of the optic plate were used in the assessment of the glaucoma clutter. Fard et al. [6] utilized a Support Vector Machine (SVM) when calculating the F-cut done through the use of random mass transformation. Among the various detection techniques, background image-based detection of the retina plays an important role as it was subjected to non-invasive detection methods. Then, they made the categorization after selecting the shape. Detecting glaucoma disorder could be achieved by determining the shape of the retina and checking various medical characteristics of the eyes, such as measurements of the thickness of the retinal nerve fiber layer, the relationship between the edge and the disc, the optic disc and the head of the nerve. Rahman et al. [7] investigated the distinct techniques of glaucoma detection using retinal base images. Sharma et al. [8] studied retinal blood vessel segmentation like glaucomatous eye image segmentation.
Soltani et al. [9] predicted glaucoma in the early stage by identifying several characteristics of glaucoma which could be represented as (i) open-tangent glaucoma, (ii) closed-angle Glaucoma, (iii) normal-tension glaucoma, and (iv) connective glaucoma. Welfer et al. [10] tested tonometry (measures the pressure within the eye), ophthalmoscopy (inside the fundus of the eye and other structures), perimetry (systematic calculation of the visual field), gonioscopy (anatomical angle developed between the eye’s cornea and iris) and pachymetry (measures the thickness of the eye’s cornea) and noted that these tests need to be conducted to detect glaucoma. Recently, the detection of glaucoma has been achieved using Random-Forest classifiers with a 91% accuracy. Blurred convergence of inbreeding glaucoma was used to locate the optical resolution in the retinal frame. A test for glaucoma was performed using a digital eye camera to check for optical coherence tomography (OCT), measurement of intraocular pressure (IOP), and evaluations of optic nerve hypoplasia (ONH), retinal nerve fiber layer (RNFL), and visual field defects [11].
The SVM is a supervised machine learning algorithm that can be used for both classification and regression challenges which can be utilized in the process of glaucoma detection [12]. This method is based on the adaptive threshold and it improves the image quality by removing the noises in the image. On retinal images, Li et al. [13,14] and Vostatek et al. [15] proposed vessel segmentation and amplitude estimation utilizing large-scale production of matching filtered responses.
Gour et al. [16] reported an approach to detect the order of glaucoma using eye-based images. This was done through a computerized diagnostic system that was used to find the malformation present in the fundus images. Fraz et al. [17] developed the method of perceiving glaucoma progression using Heidelberg Retinal Tomography (HRT) images. Fuzzy differential equations (FDE) along with intuitionistic fuzzy sets (IFS) are used to develop reliable traffic management systems and shift scheduling for supporting an automatic transmission along with artificial intelligence (AI). In robotics, FDE brings changes in the navigational directions to avoid the obstacle. Furthermore, it can be used in finding the difference between the crossover rate and mutation rate in the genetic algorithmic optimization process.
For a fixed set
Here
Here, Eqs. (1) and (2) are used to identify the max-min (
where S, D, and P are the finite sets of symptoms, type of disease, and patients, respectively. Fuzzy Relations (FR) are defined as
where
Based on the max–min consensus convergence algorithm, each stage was iteratively updated according to its weighted average together with its minimum and maximum stages from early stage to glaucomatous eyes [21].
From
(i)
(ii)
It was also applied to the three aforementioned categories, including glaucomatous, suspected to be glaucomatous, and non-glaucomatous [22]. This new measure
3.1 Image Enhancement Using Fuzzy
Some numerical specifications were required to enhance the image. Here, we considered the main CDR, optic nerve head, and area of optic nerve values. In general, CDR ranged from 0.26 to 0.5 mm. The average optic nerve head diameter varied from 1.2 to 2.5 mm. The area of the optic nerve head in
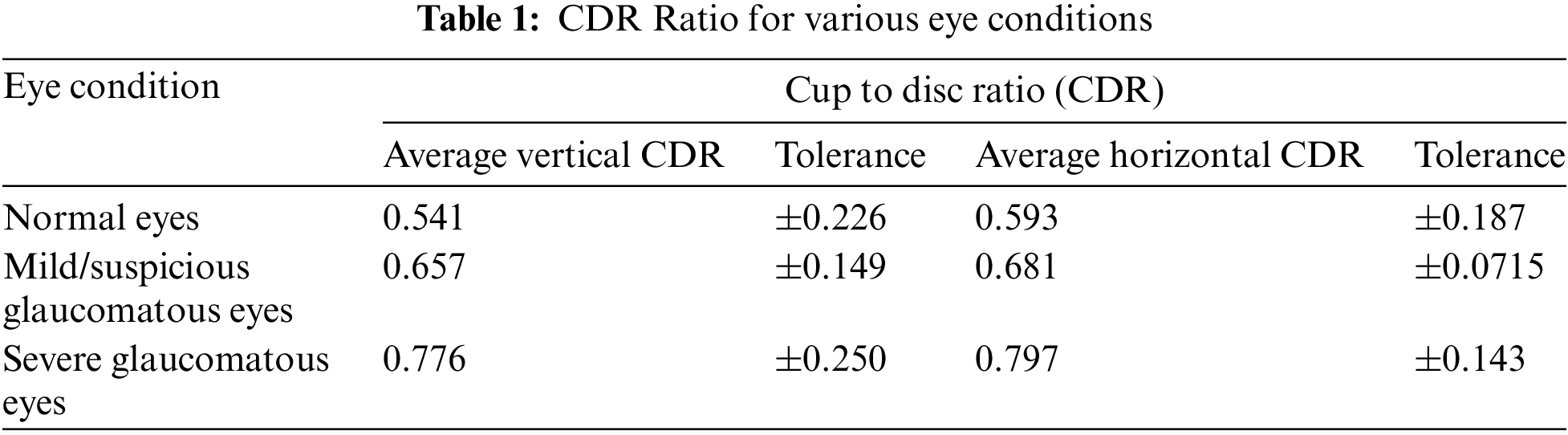
The predicted retinal image’s cup to disc values for average vertical CDR ranged from 0.541 (±0.226) to 0.593 (±0.187) for average horizontal CDR. The retinal image’s cup to disc values for suspected glaucoma ranged from 0.657 (±0.149) for vertical CDR to 0.681 (±0.0715) for horizontal CDR. For glaucomatous eyes, the vertical CDR range was 0.776 (±0.250), and the horizontal CDR was 0.797 (±0.143) as shown in Table 1.
Let us consider a retinal image
where
where
3.2 Fuzzy Difference Equation Computation to Detect Glaucoma in Retinal Images
The first step for fuzzy computation of the retinal image
The membership degrees of the three fuzzy sets (three images to be analyzed) described by using Eqs. (12)–(14) must be added and equated with one in accordance with the partition definition. Consequently, the fuzzy degrees of belonging together with the weighted values
Then, an instruction parameter
The fuzzy degrees of belonging, together with the weighted values
Now, the fuzzy weighted regularization could be calculated for the second image using the attenuation parameter
Similarly, the fuzzy degrees of belonging together, along with the weighted values
The three sets of calibrated weighted fuzzy partitions that use the attenuation parameter
After changing the membership degree, the membership gradation for the sets
A similar approach was taken to obtain
The value Λ could be added from the modified fuzzy partition sets using Eqs. (20) and (21) as follows:
A similar approach was followed for
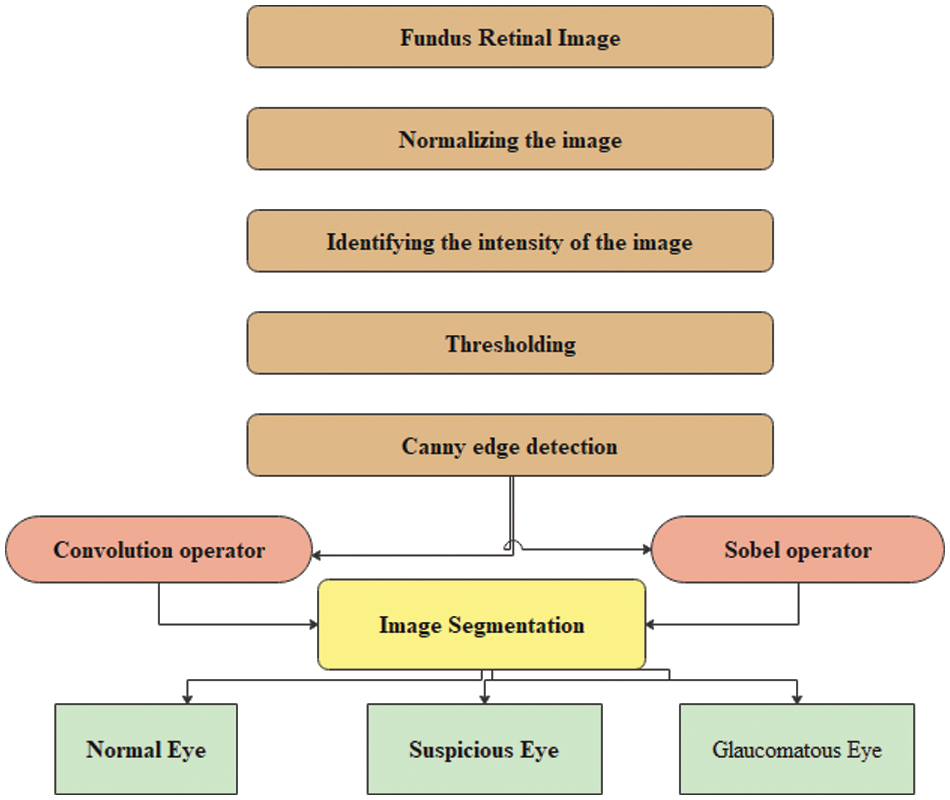
Figure 1: Proposed CNN algorithm for classifying glaucomatous eyes
The selection of the new and modified degree of membership for each fuzzy series of the three partitions based on
Eq. (24) provided a basis for the membership degrees
3.3 Fuzzy Difference Equation Manipulation and Defuzzification
Fuzzy manipulation functions were obtained by using Eq. (20) to improve the contrast of the retinal image.
The enhanced retinal images represented for each of the three images by
where
4 Image Segmentation Using Fuzzy Differential Equations
Fuzzy integrals based on the method of combining a fuzzy image region with different fuzzy characteristics have been used in nonlinear image processing (region, edge detection, histogram, and analyzer) for segmentation. These are used to recursively combine the regions based on the maximum and minimum fuzzy integral [26]. The goal of integrating the regions is to combine the fuzzy targets, and evidence of the fuzzy measurement is reflected in the image regions. The proposed retinal image processing algorithm is shown in Fig. 2 and the implementation of the proposed algorithm was achieved as depicted in Figs. 3–5.
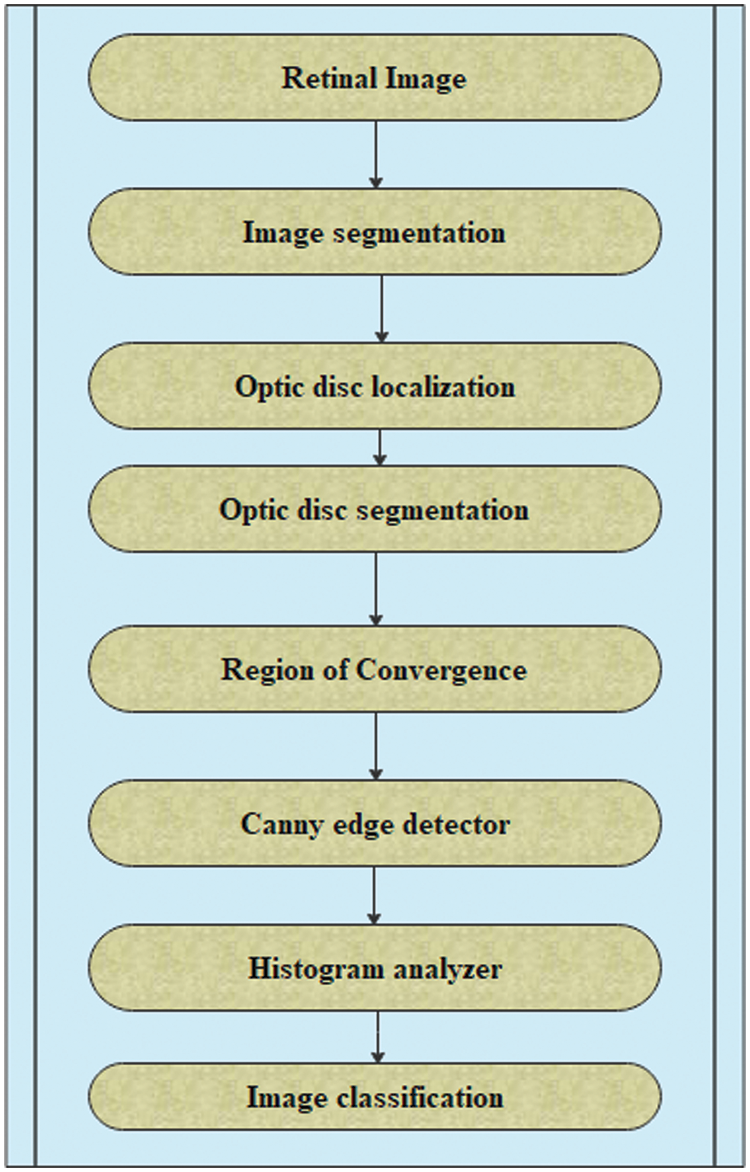
Figure 2: Algorithm for retinal image processing
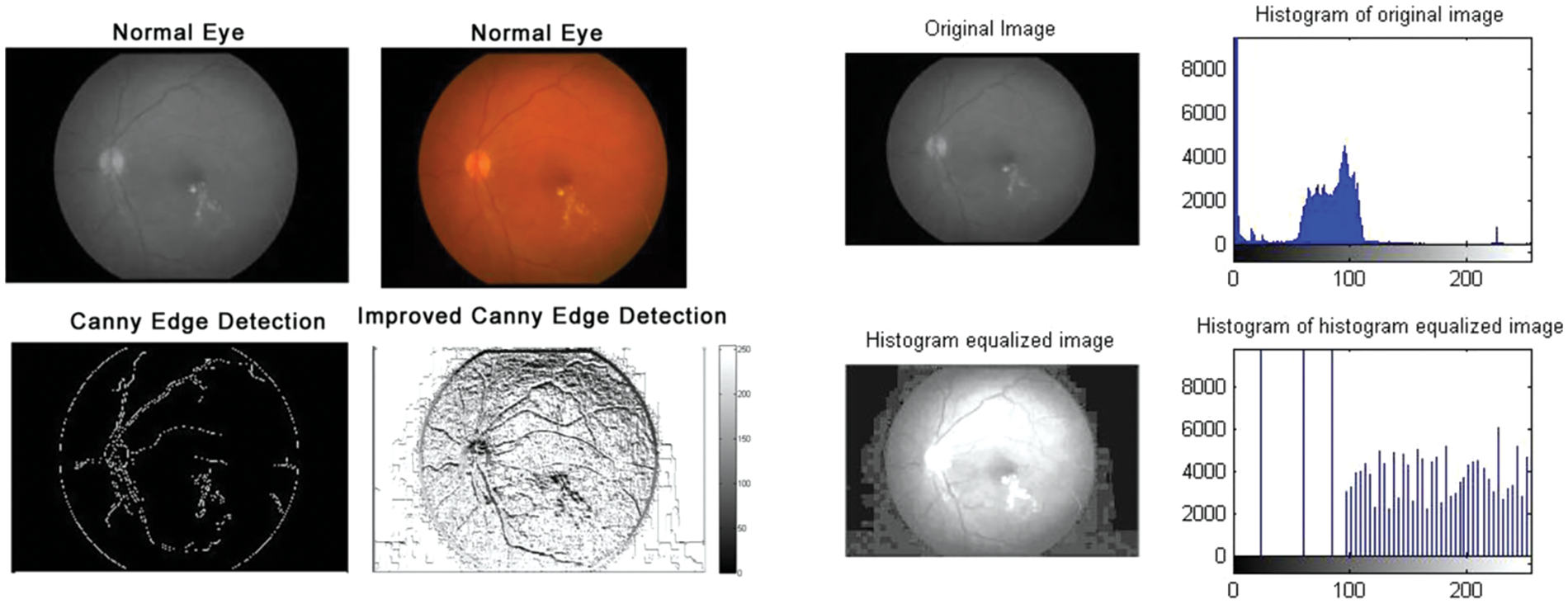
Figure 3: Normal eyes: Edge detection and histogram of histogram equalized image
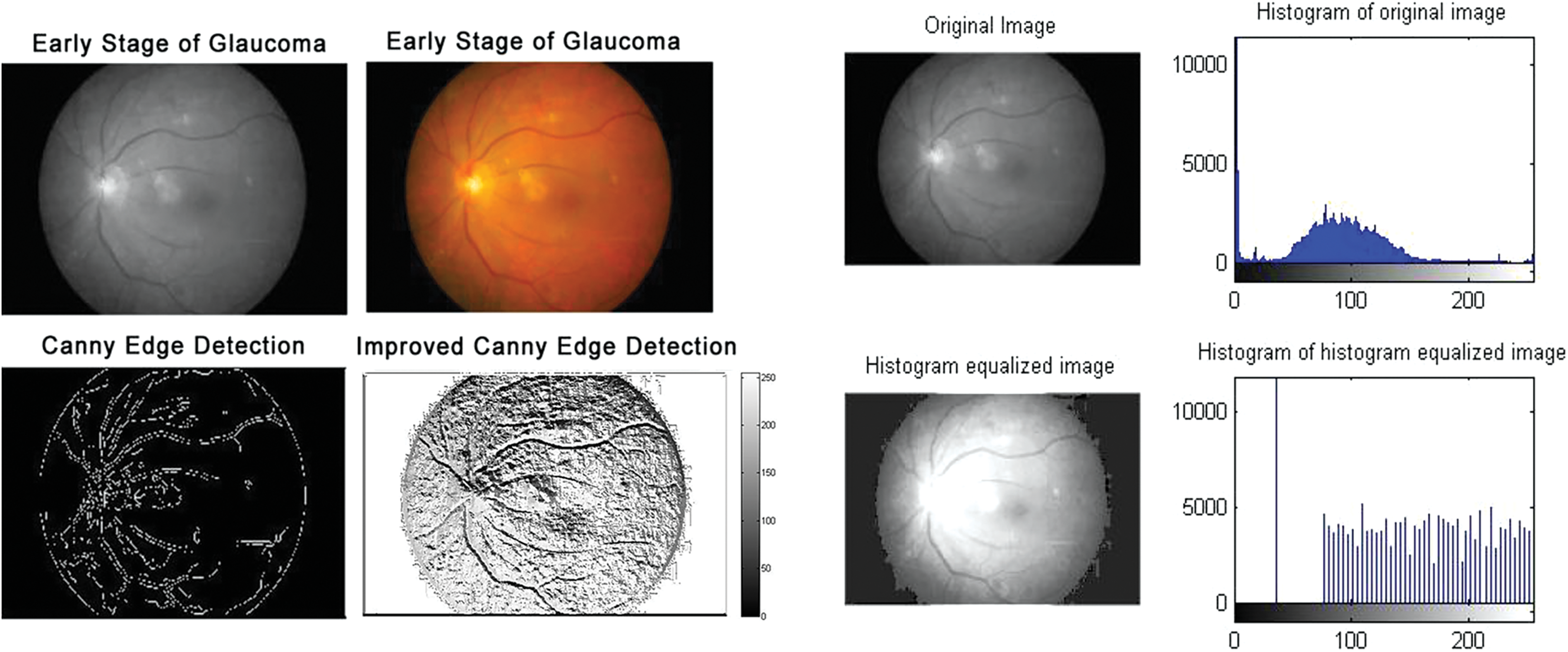
Figure 4: Early-stage glaucomatous eyes: Edge detection and histogram of histogram equalized image
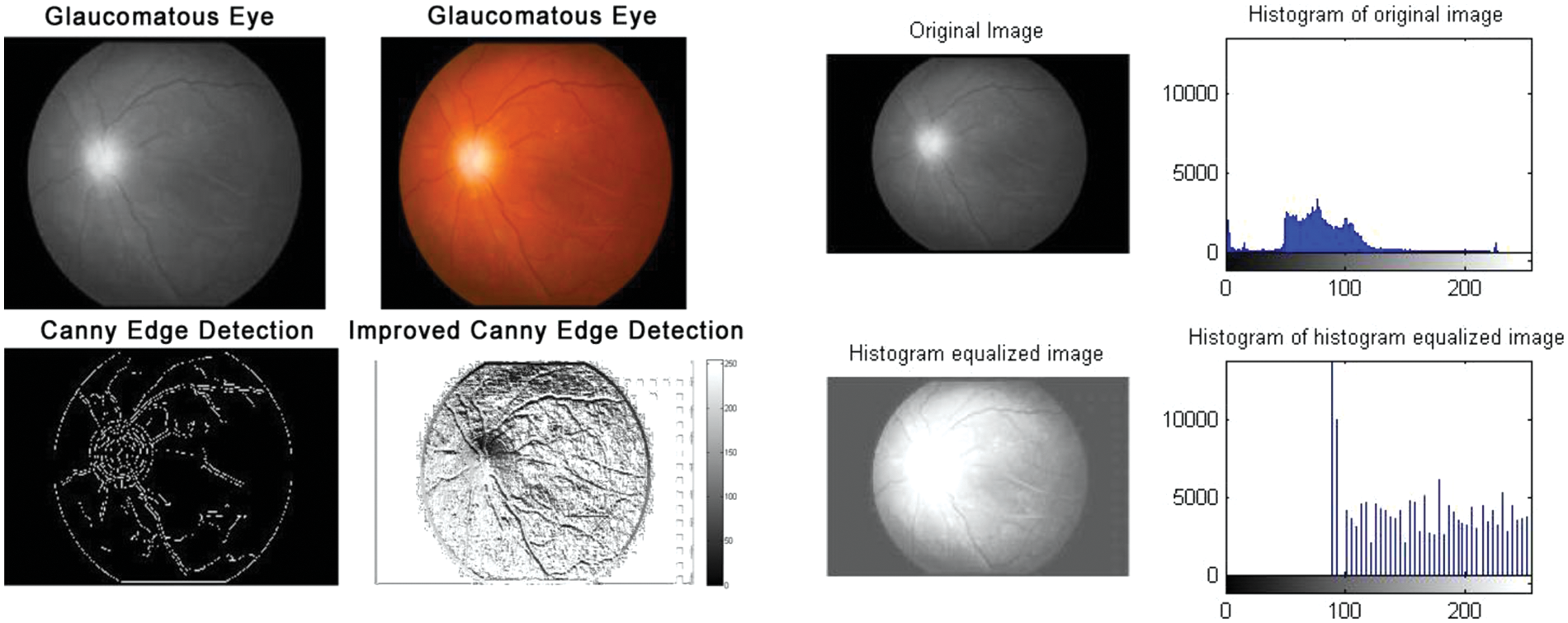
Figure 5: Glaucomatous eyes: Edge detection and histogram of histogram equalized image
Let
The calculation of fuzzy integral for image segmentation in Eq. (22),
4.1 Fuzzy Differential Equations’ Calculations
The predictable three fuzzy partitions sets were defined as
The three fuzzy density values were estimated to be
Now, the characteristic of image δ could be obtained using the three characterized fuzzy densities
Therefore, any of the skills in the set of measures
After measuring the fuzzy density in the set of {a0, a1, a2}, the measurable function
The above three equations must satisfy
where
The degree of belonging could be classified as an object in two classes
The degree of belonging could be classified as an object in two classes
Therefore, an object could belong to the class
where ζ is defined as ζ
The segmented retinal image
where
According to the proposed algorithm, which is illustrated in Figs. 1 and 2, the following were applied to the sample images. Canny edge detection, histogram equalized images, histogram of original images, and histogram of histogram equalized images were classified for normal eyes, early-stage glaucomatous eyes, and glaucomatous eyes as shown in Figs. 3–5.
To assess objective improvement, we used the fuzzy spatial frequency metric along with a canny edge detector to analyze the execution of the proposed strategy towards the parameters of the area of the disc of the age group of 30–40-year-olds and 41–50-year-olds. The corresponding disc diameters (both horizontal and vertical discs), temporal inferior, temporal superior, and nasal were taken as parameters. Sample data for 20 individuals were analyzed and the results are presented in Table 2 and illustrated in Fig. 6.
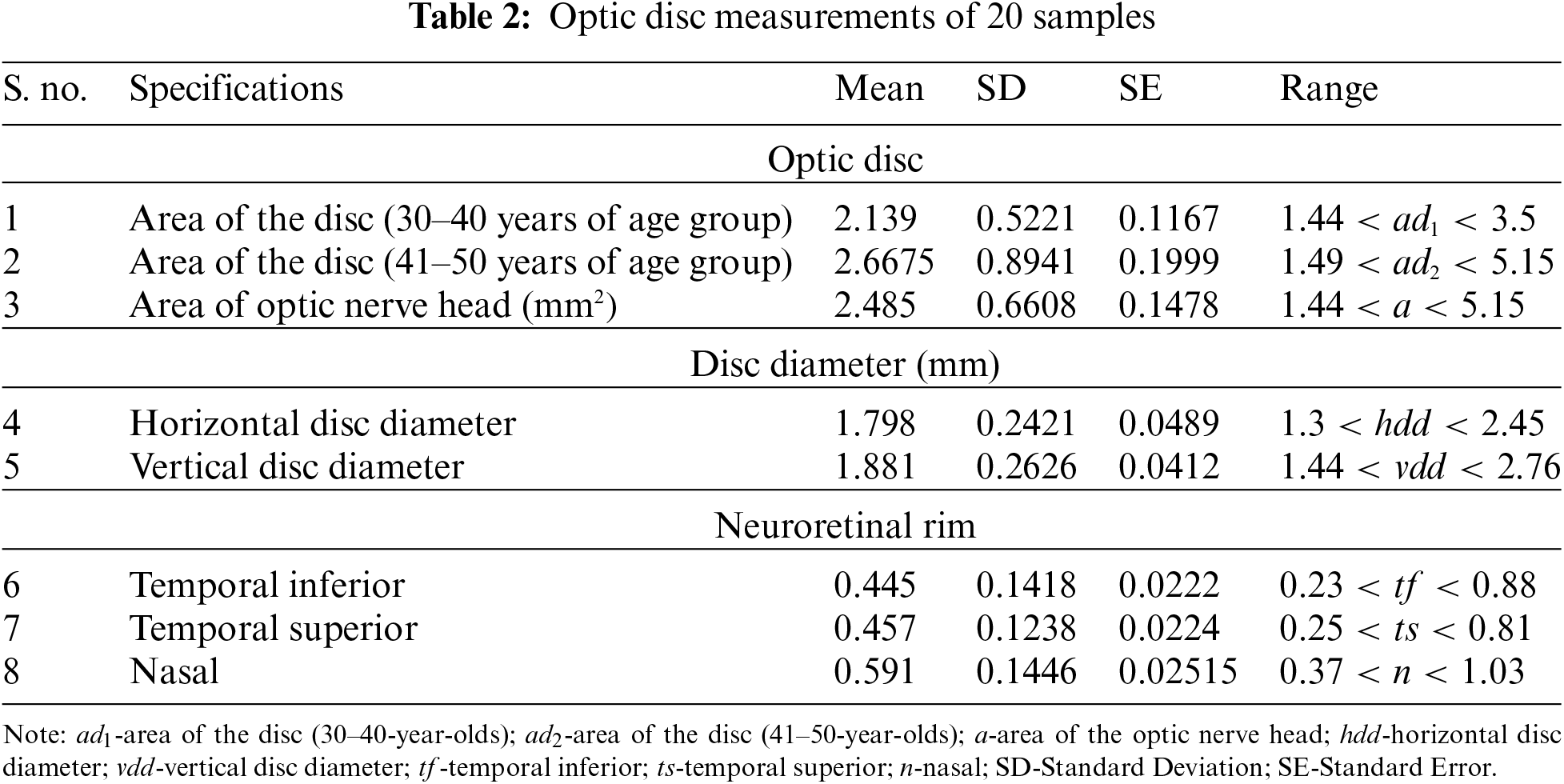
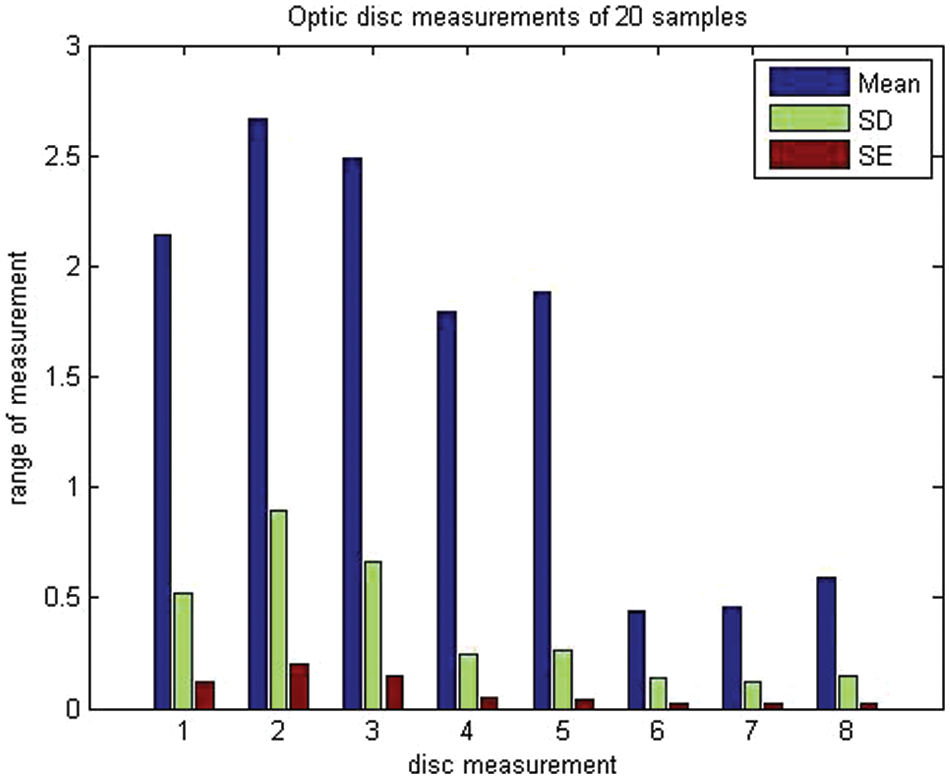
Figure 6: Mean, standard deviation, and standard error of optic disc measurements of 20 samples describing their mean, standard deviation and standard error
In general, according to the algorithm of retinal image processing which was shown in Fig. 2, the spatial recurrence was assessed based on the standard level of visual clarity. When the spatial recurrence was determined to be a high value, which indicated better vision,
where R represents the row frequency (a horizontal measurement of CDR) and C represents the frequency column (a vertical measurement of CDR),
Similarly,
Thus, Eqs. (37)–(39) along with Eqs. (24)–(26) could be used to categorize the eye condition of the patient. The validation of this categorization was then validated through hypothetical medical information, such as optic disc area, disc diameter, and neuroretinal rim values. Row and column frequencies were also used to obtain and classify the range of the region of convergence of the retinal images.
A novel approach for the detection of a glaucomatous eye using intuitive fuzzy sets with membership and non-membership functions was discussed in this study. This was accomplished by utilizing intuitive fuzzy (optimal classifier) sets in the region of convergence of the partitioned retinal images. Fuzzy degrees and fuzzy weighted values were then used as the concentration level in the retinal images. Once the concentration levels of the retinal images were found, the image was then classified as normal eyes, suspected glaucomatous eyes, or glaucomatous eyes. The measurement of the concentration level and checking for similarities in different regions of the images generated a threshold image from the combined fuzzy matrix along with the spatial frequency fuzzy metric which led to the precise classification of the eye condition. Canny edge detection, a histogram equalized image, a histogram of original images and a histogram of histogram equalized images were classified for normal eyes, early-stage glaucomatous eyes, and glaucomatous eyes. These techniques were assorted through the proposed deep learning algorithm using the Convolutional Neural Network. Based on this analysis, the intuitionistic fuzzy sets were carried out on a similar pattern identifier envisaged by models of human perception.
Acknowledgement: The authors express their appreciation to King Saud University for funding the publication of this research through the Researchers Supporting Program (RSPD2023R809), King Saud University, Riyadh, Saudi Arabia.
Funding Statement: This research was funded by the Researchers Supporting Program at King Saud University (RSPD2023R809), Riyadh, Saudi Arabia.
Author Contributions: Dorathy Prema Kavitha, L. Francis Raj, Sandeep Kautish and Abdulaziz S. Almazyad conceived of the presented idea. D. Dorathy Prema Kavitha and L. Francis Raj developed the theory and performed the computations. Sandeep Kautish, Abdulaziz S. Almazyad, Karam M. Sallam, and Ali Wagdy Mohamed verified the analytical methods. Sandeep Kautish, Abdulaziz S. Almazyad encouraged D. Dorathy Prema Kavitha and L. Francis Raj to investigate the proposed method and supervised the findings of this work. All authors discussed the results, contributed to, and approved the final manuscript.
Availability of Data and Materials: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Tjandrasa, H., Wijayanti, A., Suciati, N. (2012). Optic nerve head segmentation using hough transform and active contours. Telkomnika, 10(3), 531–536. https://doi.org/10.12928/telkomnika.v10i3.833 [Google Scholar] [CrossRef]
2. Zhang, M., Chen, Y., Lin, J. (2021). A Privacy-preserving optimization of neighborhood-based recommendation for medical-aided diagnosis and treatment. IEEE Internet of Things Journal, 8(13), 10830–10842. https://doi.org/10.1109/JIOT.2021.3051060 [Google Scholar] [CrossRef]
3. Haubecker, H., Tizhoosh, H. R. (1999). Fuzzy image processing. In: Computer vision and applications. New York: Academic Press. [Google Scholar]
4. Dehghani, A., Moghaddam, H. A., Moin, M. S. (2012). Optic disc localization in retinal images using histogram matching. EURASIP Journal on Image and Video Processing, 2012(1), 19. https://doi.org/10.1186/1687-5281-2012-19 [Google Scholar] [CrossRef]
5. Naik, S. K., Murthy, C. A. (2003). Hue-preserving color image enhancement without gamut problem. IEEE Transactions on Image Processing, 12(12), 1591–1598. https://doi.org/10.1109/TIP.2003.819231 [Google Scholar] [PubMed] [CrossRef]
6. Fard, O. S., Bidgoli, T. A., Rivaz, A. (2017). On existence and uniqueness of solutions to the fuzzy dynamic equations on time scales. Mathematical and Computational Applications, 22(1), 16. [Google Scholar]
7. Rahman, G., Din, Q., Faizullah, F., Khan, F. M. (2018). Qualitative behavior of a second-order fuzzy difference equation. Journal of Intelligent and Fuzzy Systems, 34(1), 745–753. [Google Scholar]
8. Sharma, S., Wasson, E. V. (2015). Retinal blood vessel segmentation using fuzzy logic. Journal of Network Communications and Emerging Technologies (JNCET), 4(3), 1–5. [Google Scholar]
9. Soltani, A., Battikh, T., Jabri, I., Lakhoua, N. (2018). A new expert system based on fuzzy logic and image processing algorithms for early glaucoma diagnosis. Biomedical Signal Processing and Control, 40, 366–377, https://doi.org/10.1016/j.bspc.2017.10.009 [Google Scholar] [CrossRef]
10. Welfer, D., Scharcanski, J., Kitamura, C. M., Dal Pizzol, M. M., Ludwig, L. W. B. et al. (2010). Segmentation of the optic disk in color eye fundus images using an adaptive morphological approach. Computers in Biology and Medicine, 40(2), 124–137. https://doi.org/10.1016/j.compbiomed.2009.11.009 [Google Scholar] [PubMed] [CrossRef]
11. Paulus, J., Meier, J., Bock, R., Hornegger, J., Michelson, G. (2010). Automated quality assessment of retinal fundus photos. International Journal of Computer Assisted Radiology and Surgery, 5, 557–564. https://doi.org/10.1007/s11548-010-0479-7 [Google Scholar] [PubMed] [CrossRef]
12. Atanassov, K. T. (1986). Intuitionistic fuzzy sets. Fuzzy Sets and Systems, 20(1), 87–96. https://doi.org/10.1016/S0165-0114(86)80034-3 [Google Scholar] [CrossRef]
13. Li, Q., You, J., Zhang, D. (2012). Vessel segmentation and width estimation in retinal images using multi scale production of matched filter responses. Expert Systems with Applications, 39, 7600–7610. https://doi.org/10.1016/j.eswa.2011.12.046 [Google Scholar] [CrossRef]
14. Li, Y., Du, L., Wei, D. (2022). Multiscale CNN based on component analysis for SARATR. IEEE Transactions on Geoscience and Remote Sensing, 60, 1–12. https://doi.org/10.1109/TGRS.2021.3100137 [Google Scholar] [CrossRef]
15. Vostatek, P., Claridge, E., Uusitalo, H., Hauta-Kasari, M., Falt, P. et al. (2017). Performance comparison of publicly available retinal blood vessel segmentation methods. Computerized Medical Imaging and Graphics, 55, 2–12. https://doi.org/10.1016/j.compmedimag.2016.07.005 [Google Scholar] [PubMed] [CrossRef]
16. Gour, N., Khanna, P. (2019). Automated glaucoma detection using GIST and pyramid histogram of oriented gradients (PHOG) descriptors. Pattern Recognition Letters, 137, 3–11. https://doi.org/10.1016/j.patrec.2019.04.004 [Google Scholar] [CrossRef]
17. Fraz, M. M., Remagnino, P., Hoppe, A., Uyyanonvara, B., Rudnicka, A. R. et al. (2012). Blood vessel segmentation methodologies in retinal images–A survey. Computer Methods and Programs in Biomedicine, 108(1), 407–433. https://doi.org/10.1016/j.cmpb.2012.03.009 [Google Scholar] [CrossRef]
18. Alamin, A., Mondal, S. P., Alam, S., Goswami, A. (2020). Solution and stability analysis of non-homogeneous difference equation followed by real life application in fuzzy environment. Sādhanā, 45, 185. https://doi.org/10.1007/s12046-020-01422-1 [Google Scholar] [CrossRef]
19. Mjahed, O., Hadaj, S. E., Mahdi, E., Mjahed, S. (2023). Improved supervised and unsupervised metaheuristic-based approaches to detect intrusion in various datasets. Computer Modeling in Engineering & Sciences, 137(1), 265–298. https://doi.org/10.32604/cmes.2023.02758 [Google Scholar] [CrossRef]
20. Sun, T., Su, G., Han, C. H., Zeng, F. P., Qin, B. (2022). Eventual periodicity of a system of max-type fuzzy difference equations of higher order. Fuzzy Sets and Systems, 443, 286–303. [Google Scholar]
21. Hoover, A., Goldbaum, M. (2003). Locating the optic nerve in a retinal image using the fuzzy convergence of the blood vessels. IEEE Transactions on Medical Imaging, 22(8), 951–958. https://doi.org/10.1109/TMI.2003.815900 [Google Scholar] [PubMed] [CrossRef]
22. Huang, M. L., Chen, H. Y., Huang, J. J. (2007). Glaucoma detection using adaptive neuro-fuzzy inference system. Expert Systems with Applications, 32, 458–468. https://doi.org/10.1016/j.eswa.2005.12.010 [Google Scholar] [CrossRef]
23. Thakur, N., Juneja, M. (2018). Survey on segmentation and classification approaches of optic cup and optic disc for diagnosis of Glaucoma. Biomedical Signal Processing and Control, 42, 162–189. https://doi.org/10.1016/j.bspc.2018.01.014 [Google Scholar] [CrossRef]
24. Rajchakit, G., Agarwal, P., Ramalingam, S. (2021). Stability analysis of neural networks. Singapore: Springer. https://doi.org/10.1007/978-981-16-6534-9Se [Google Scholar] [CrossRef]
25. Hanmandlu, M., Jha, D. (2006). An optimal fuzzy system for color image enhancement. IEEE Transactions on Image Processing, 15(10), 2956–2966. https://doi.org/10.1109/TIP.2006.877499 [Google Scholar] [PubMed] [CrossRef]
26. Hussain, S. A., Holambe, A. N. (2015). Automated detection and classification of Glaucoma from eye fundus images: A survey. International Journal of Computer Science and Information Technologies, 6(2), 1217–1224. [Google Scholar]
27. Rajchakit, G., Sriraman, R. (2021). Robust passivity and stability analysis of uncertain complex-valued impulsive neural networks with time-varying delays. Neural Processing Letters, 53, 581–606. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools