 Open Access
Open Access
ARTICLE
Optimization of Cooperative Relaying Molecular Communications for Nanomedical Applications
1 Department of Electrical Engineering, Faculty of Engineering, Minia University, Minia, 61111, Egypt
2 Department of Electronics and Electrical Communications Engineering, Faculty of Electronic Engineering, Menoufia University, Menouf, 32952, Egypt
3 Department of Informatics, Faculty of Mathematics and Natural Sciences, University of Oslo (UiO), Oslo, Norway
4 Computer Science Department, Community College, King Saud University, Riyadh, 11437, Saudi Arabia
5 Department of Computer science, The Higher Future Institute for Specialized Technological Studies, El Shorouk, Egypt
Saied M. Abd El-atty. Email:
# Corresponding Address: Hellenic Ministry of Foreign Affairs, Athens, Greece
Computer Modeling in Engineering & Sciences 2024, 138(2), 1259-1275. https://doi.org/10.32604/cmes.2023.028990
Received 22 January 2023; Accepted 10 July 2023; Issue published 17 November 2023
Abstract
Recently, nano-systems based on molecular communications via diffusion (MCvD) have been implemented in a variety of nanomedical applications, most notably in targeted drug delivery system (TDDS) scenarios. Furthermore, because the MCvD is unreliable and there exists molecular noise and inter symbol interference (ISI), cooperative nano-relays can acquire the reliability for drug delivery to targeted diseased cells, especially if the separation distance between the nano transmitter and nano receiver is increased. In this work, we propose an approach for optimizing the performance of the nano system using cooperative molecular communications with a nano relay scheme, while accounting for blood flow effects in terms of drift velocity. The fractions of the molecular drug that should be allocated to the nano transmitter and nano relay positioning are computed using a collaborative optimization problem solved by the Modified Central Force Optimization (MCFO) algorithm. Unlike the previous work, the probability of bit error is expressed in a closed-form expression. It is used as an objective function to determine the optimal velocity of the drug molecules and the detection threshold at the nano receiver. The simulation results show that the probability of bit error can be dramatically reduced by optimizing the drift velocity, detection threshold, location of the nano-relay in the proposed nano system, and molecular drug budget.Keywords
Following the COVID-19 crisis with the increase of severe diseases, and the shortage of medical specialists, new healthcare monitoring technologies are required. One of these technologies is the nano-system (NS) for infectious disease diagnosis [1–3]. It can also be used to deliver therapeutic drugs to the targeted tissue. Furthermore, such nano-system networks can be remotely controlled and monitored using the Internet of Bio-Nano Things (IoBNT) technology [4,5]. The IoBNT is a broad paradigm for remotely managing a biological network of nanosystems, and thus interfacing the biological network with external networks such as the Internet [6,7]. The NS is made up of a collection of nanomachines such as nano-transmitter, nano-relay and nano-receiver that artificially mimic the biological cell [8]. Communication between nanomachines is accomplished through molecular communication via diffusion (MCvD). The MCvD has captured a particular interest due to its energy effectiveness and bio-compatibility. The molecular information in MCvD is encoded using a variety of methods, including molecular concentration, time, and molecular type [9]. In this study, we consider a hybrid encoding of molecular information by the emitted concentration and the molecular type. Additionally, in MCvD, the nano-transmitter releases drug molecules in an aqueous medium. They randomly walk in all available directions according to Brownian motion [10]. Subsequently, due to the randomness of the movement of molecules, a lot of therapeutic molecules cannot reach the nano-receiver, especially if the separation distance between the nano-transmitter and the nano-receiver is large. In flow-based diffusion, the drug molecules are restricted in the micro-fluid channels as blood vessels [11]. Therefore, molecular communication suffers from high propagation delay in proportion to the square of the distance between the nano-transmitter and the nano-receiver. Moreover, the concentration of molecules is inversely proportional to the cubic power of the separation between the nano-transmitter and the nano-receiver. These characteristics result in communication unreliability and deterioration between the nano-transmitter and the nano-receiver [12,13]. These problems can be solved using relay capabilities. Relay strategies can be found in biological systems, such as the nervous system and quorum sensing in bacteria [14].
As nano-relaying plays an essential role in nano-networks based on diffusive molecular communication, various research efforts have been presented on flow-based diffusive molecular communication, relay molecular communication and optimization criteria. In [15], a mobile multiple-input multiple-output system for MCvD was introduced by combining decode-and-forward and network coding at relay nano-machines. The authors defined the thresholds at the relay and destination nanomachines by the maximum a posteriori (MAP) probability detection algorithm. A new network coding algorithm was introduced in [16] for mitigating ISI and enabling a larger-range communication. The half-duplex network coding is used for sending the nanomachine symbols to the nano-relay. A flow-induced diffusive channel model was investigated in [17] to study the effect of velocity with different mobility conditions. The performance of the molecular communication system with on-off keying (OOK) modulation was evaluated in terms of bit error rate (BER). A decision rule and an adaptive decision threshold have been derived using the likelihood ratio. A two-hop molecular communication system was introduced in [18]. The authors considered molecular degradation and noise effect over channel. They used a joint optimization method for resource allocation and position determination of relays with given detection thresholds to reduce BER. In [19], a nano-relay has been presented between the sender and destination nodes. It combines the collected portion of molecules released from the sender and stores them for a fraction of time, and then they are released to the receiver. Thereby, the delayed and non-delayed received molecules arrive at the same time, and thus the signal strength is improved. The most accurate vitro biological barrier models created to study the dynamic interaction of nanoconstructs for diagnostic or therapeutic purposes in this constantly changing environment are summarized by the authors of [20]. They assisted nanotechnologists in selecting the best vitro models for their specific experimental needs. The advantages of the most popular drug-carrier system liposomes were discussed by the authors of [21]. They investigated the effects of size, surface charge, and lipid structure on the efficiency of liposomes as well as their composition. The impact of liposome physicochemical characteristics on in vivo cell contact, half-life, tissue infiltration, and final state was also examined. Along with liposome-based drugs that are currently available in clinical trials, the authors also considered a number of strategies that have been created to get around the drawbacks of liposomes’ first generation. On the other hand, partial differential equations were used in [22] to model the fluids’ flow and temperature behavior. In order to study natural heat convection inside square and equilateral triangular cavities, a meshless approach based on a collocation of local radial basis functions was used. To assess the system precision, a number of natural convection test cases in square and triangular cavities were selected. Numerous academic papers examined the chemical and physical characteristics of natural molecular aspects as in [23].
As illustrated by the aforementioned literature, these systems may result in solutions to the problems in MCvD. However, optimization has not been considered widely in engineering aspects of cooperative molecular communication systems. Effective and robust computational algorithms are required to solve optimization problems that arise in such systems. Several global optimization algorithms have recently been presented such as particle swarm optimization (PSO) which is based on swarm movement, and MCFO which is subject to the law of gravity [24,25]. The PSO is simple and effective, with few parameters. The MCFO, on the other hand, is relatively complex, but it is capable of optimizing multiple parameters with high performance [26,27].
Unlike previous literature [15], [18], [28] and [29], this paper presents a new optimization technique that can work on multiple parameters. In the related literature, the drift velocity is the most important parameter. In this work, we investigate the cooperative nano-relay problem in MCvD with drift flow velocity. To improve the reliability of delivering drug molecules from the nano-source to the nano-receiver, a decoding nano-relaying scheme is used. We developed a cooperative decoding nano-relay based on flow velocity to deliver predetermined therapeutic drug molecules with a carefully determined drift velocity (optimized drift velocity) to the targeted cell. Furthermore, we adopt the energy detection method as the diversity combining technique to solve the detection problem at the cooperative nano-relay. The MCFO optimization technique is used to optimize the nano-network parameters for minimizing the probability of error. We believe that the proposed nanonetwork should be implanted in the human body as a form of therapy to effectively deliver therapeutic drug molecules to diseased cells with a carefully-chosen effective velocity. The basic contributions of this work are as follows:
• Analyzing a nano-system based on a cooperative nano-relaying scheme that depends on drift velocity in molecular communication.
• Deriving the bit error probability of the proposed detection method, in which the received signals from the cooperative paths are merged linearly at the nano-receiver.
• Developing an optimization technique, namely MCFO, which is based on the block coordinate descent algorithm (BCDA) in order to obtain the optimal parameters (drift velocity for injection of drug molecules, detection threshold, optimal position of nano-relay and resources allocated for the emitting nano-source) to minimize the bit error probability.
The rest of this work is organized as follows. Section 2 describes the proposed nano-system based on drift velocity in molecular communication, including an analysis of the cooperative nano-relay scheme. It also presents a performance analysis of the detection method. The MCFO optimization technique is also introduced in Section 2. The numerical and simulation results are presented in Section 3. Finally, Section 4 gives the concluding remarks.
We consider a scenario of implanting a cooperative nano-system in the intra-body area network (IBAN) based on the advanced achievements of nanotechnology in nanomedical applications. Furthermore, we believe that the nanonetwork should be planted close to the diseased cell to prevent drug molecules from spreading throughout the body, and thus reducing side effects in healthy cells. The aim of the proposed model is to deliver therapeutic drug molecules to the targeted cell. Fig. 1 indicates that the proposed nano-system model consists of a nano-source
• At the beginning of the
• The diffusion channel is prone to errors due to noise and ISI at each nano-machine.
• Nano-relay R receives the information molecules and decodes them. The decoded information bits are then forwarded by R to the nano-receiver D, at the
• The diversity technique is used to improve the performance of the proposed nano-system on the two different combined signals at the nano-receiverD. The two signals come from the S−R−D and the S−D paths.
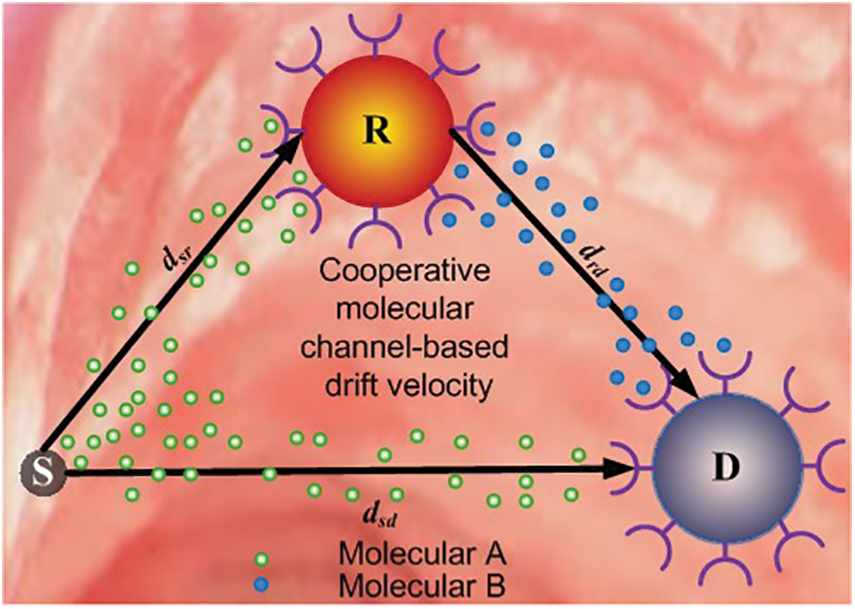
Figure 1: Cooperative molecular communication relaying scheme model based on drift velocity
As previously stated, the proposed nano-system is implanted in the human body, and thus the diffusion of therapeutic drug molecules in blood flow is based on positive drift velocity. According to Fick’s second law, the time it takes for any therapeutic drug molecule of type A released by the S to reach the R or D after the drifting distance,
where
The cumulative distribution function in Eq. (2) depends on drift velocity, diffusion coefficient, time and distance.
A cooperative molecular communication is presented as a relaying scheme based on drift velocity analysis and its BER analytical expression in the following subsections.
2.1 A Cooperative Molecular Communication Relaying Scheme Based on Drift Velocity Analysis Model
We consider a linear combination of the two received signals from the two paths, namely the first path
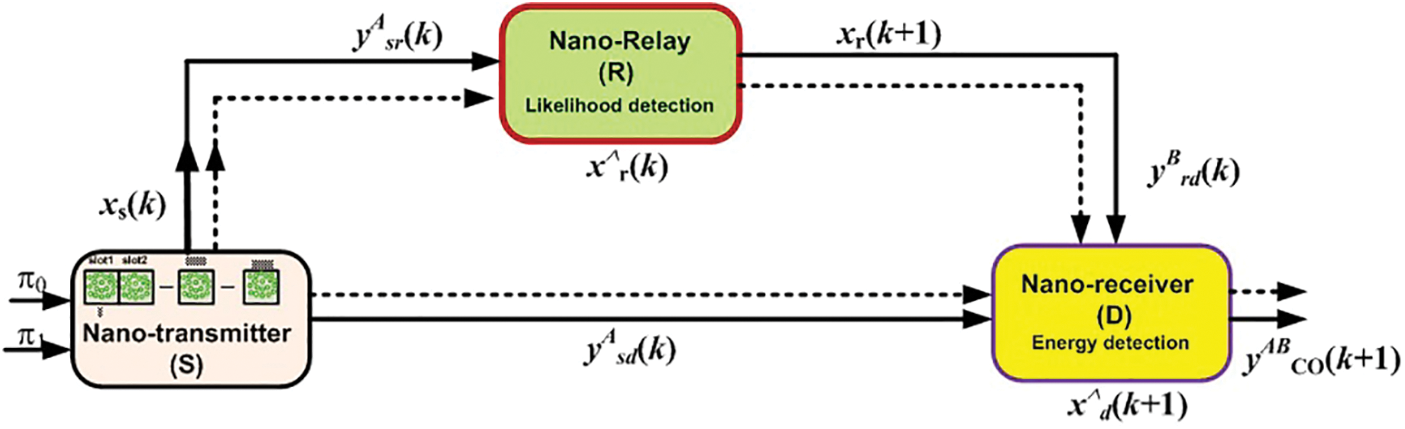
Figure 2: Schematic diagram of the proposed scheme
In the S− R−D path, let
where I is the length of ISI,
The counting noise
where
where
where the mean and variance are calculated from Eq. (8) as follows [28]:
where
The detection threshold at the nano-machine R is denoted by
As equations for the absorbed molecules for type A by the nano-machine R are given in Eqs. (3)–(8), we can derive equations for type B molecules absorbed by the nano-receiver D at the
where the mean and variance values are given by Eqs. (11)–(14) with
where
where erf(.) is the error function.
In the S−D path, the transmission of drug molecules is similar to the transmission of drug molecules in the S−R path, but with different parameters. Thereafter, the number of type A molecules collected by the nano receiver D along the S−D path in the
where the mean and variance values given by (11)–(14) can be calculated with the parameters:
2.2 Performance Analysis of the Detection Method
Energy detection is used as a diversity combining technique in the detection process. The diversity combining techniques in molecular communication cooperative networks based on drift velocity can be applied to enhance the performance of detection at the nano-receiver D. The linear combination technique gathers the received energy signals that come from the S−D and R−D paths with different types of molecules A and B in different time slots. Therefore, we can detect the signal with the next decision rule:
where
The received signals from the two paths are merged as cooperative values with equal gain combining in different time slots as shown in Fig. 2.
where the mean values can be obtained as:
where
Finally, the total probability of error for the overall system can be defined as follows [28]:
The preceding section obtained a closed-form expression of the error probability of the proposed cooperative relaying nano-network with drift velocity. To achieve the best system performance, we must first obtain the optimal solution for the most critical parameters such as drift velocity
Then, we optimize the relay position and resources allocated to S and R nodes with fixed optimal
where
This study aims to find the optimum drift velocity
2.4 Original Central Force Optimization (CFO) Algorithm
The CFO algorithm is a particle optimization algorithm that follows the gravity law. Each particle attracts every other particle with the virtual gravity force. The CFO is initialized by user-specified probe positions and acceleration distributions as follows:
The position vector of each probe is updated using the equation below:
where
2.4.1 Modified Central Force Optimization (MCFO)
The MCFO technique is a new smart optimization tool, which fixes the drawbacks of the CFO. That is, CFO occasionally halts progress towards the global optima, even when the population does not converge at the local optima or any other point. In other words, the objective of this development or modification was to enhance the global search during the early parts of the optimization and to lead the probes to converge towards the global optimum at the end of the search.
The pseudo-code of MCFO is given below. It depicts the primary steps of the MCFO algorithm, which are initialized with probe places and acceleration distributions chosen by the user on the points, which represent the
where
U(.) is the unit step function to avoid the probability of negative mass results. If negative masses were permitted, the resulting accelerations would be repulsive rather than attractive. The MCFO exponents are represented by
where
where
As mentioned before, some roles may fall outside the DS, and the MCFO may look in areas other than the DS. Hence, we introduce a simple deterministic repositioning strategy for avoiding an unallowable search space and correcting infeasible solutions. If the probe
However, if the probe
where
The capabilities of global and local search, which affect MCFO can be balanced by by updating
The steps of the MCFO pseudo-code are recorded below:
Step 1Initialization
Step 2 Compute initial probe distribution
Step 3 Compute initial fitness matrix, and select the best probe fitness
Step 4 Assign initial probe Position & acceleration
Step 5 For
Step 5.1 Compute
Step 5.2 Update probe position (35)
Step 5.3 Update probe gravitational
Step 5.4 Retrieve errant probe using (36) and (37)
Step 5.5 Update fitness matrix, and select the best probe fitness
Step 5.6 Compute probe accelerations using (33)
Step 5.7 Shrink decision space matrix by updating
Next J
Find the final solution
3 Numerical Results and Discussions
The performance of the proposed cooperative nano-system in MCvD with positive drift velocity is presented in this section. In fact, we used numerical analysis to demonstrate the proposed nanosystem performance in terms of the probability of error. We also compared the results to those of the direct link case, also known as the direct link relay (DLR) scheme presented in [29]. All of our numerical results are generated with MATLAB code and the parameters are listed in Table 1 [29].

Fig. 3 shows the performance of the probability of error in the proposed nano-system against the drift velocity of molecules with variable symbol duration
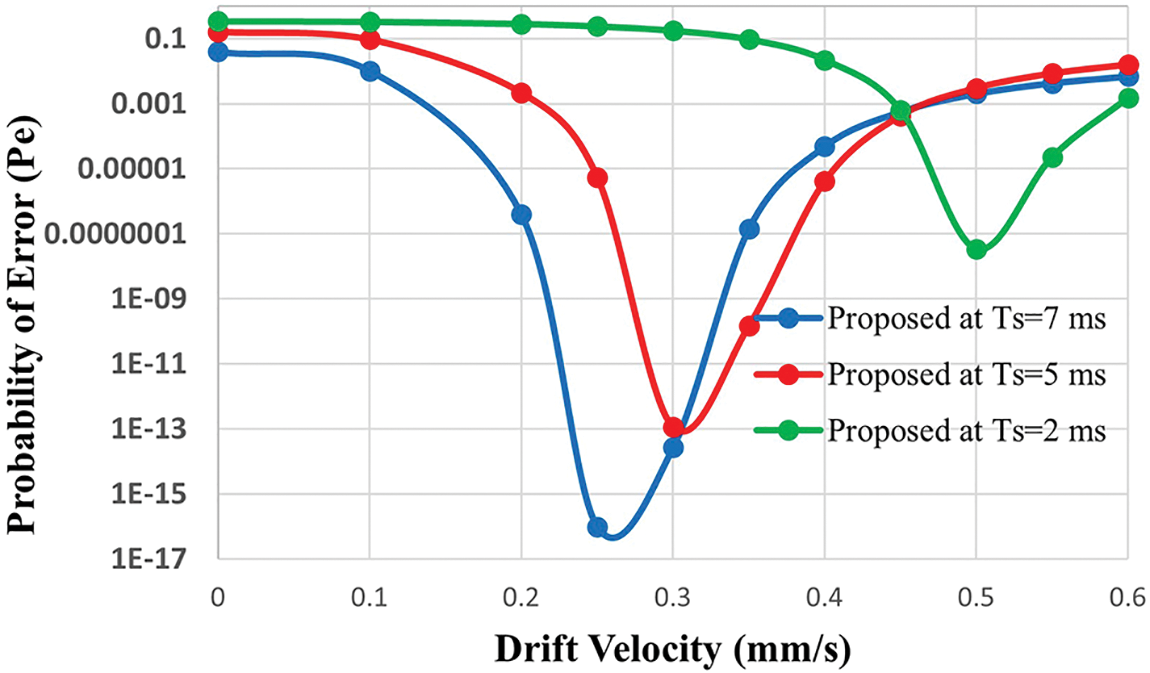
Figure 3: Probability of error of the proposed cooperative scheme as a function of drift velocity (
Fig. 4 shows the performance of the proposed nano-system and the DLR [29] in terms of bit error probability vs. detection threshold, when the drift velocity of molecules is fixed at
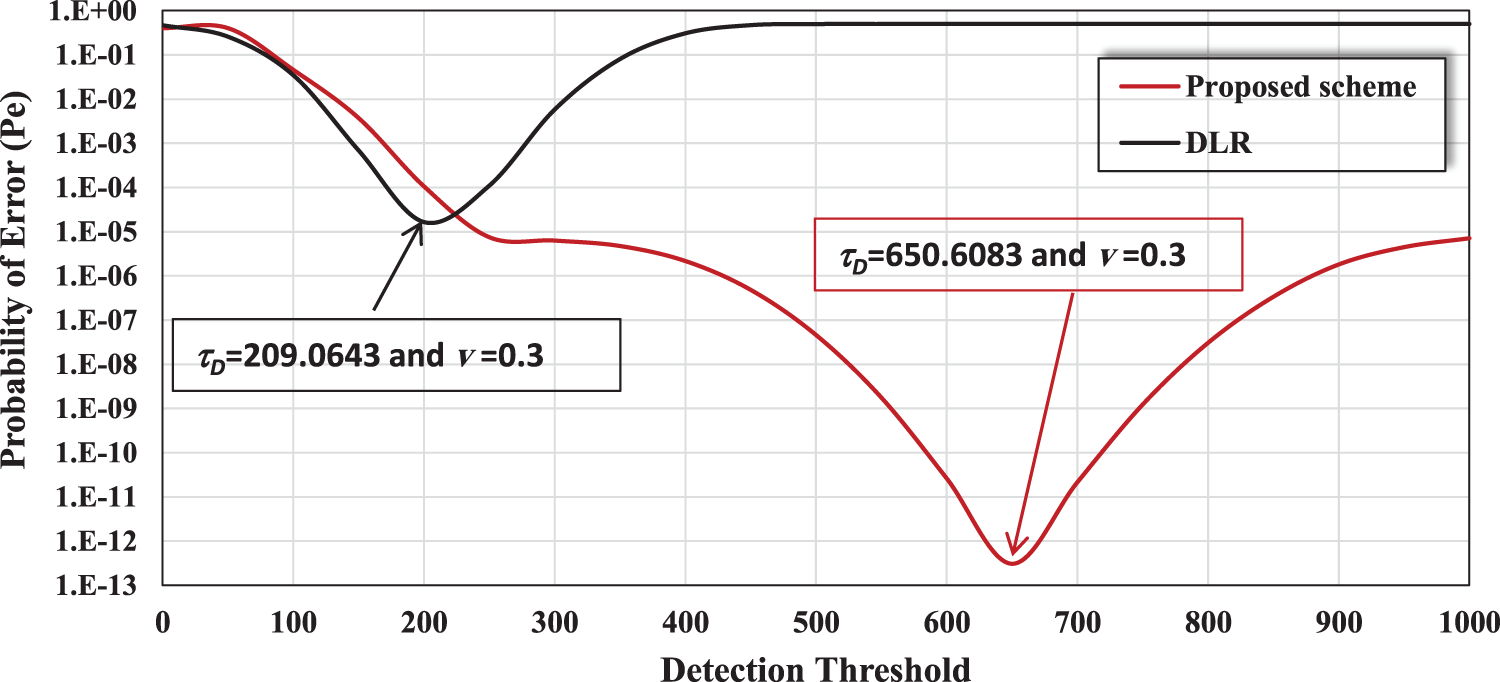
Figure 4: Comparison of the proposed scheme and the DLR scheme in terms of probability of error (
Table 2 shows the performance of the probability of error, when employing the proposed nano-system and the DLR system against the molecular budget of types A and B. We consider S and R nano-machines releasing equal molecular budget. The performance of the probability of error is depicted with optimized parameters

We observe that the proposed cooperative nano-system with the nano-relay based on a diversity combining technique expends a lower molecular budget, and thus a significant increase in nano-system performance is achieved compared with the DLR system. For example, at
Figs. 5 and 6 show the joint optimization of nano-relay position and molecular budget for S and R nanomachines with a fixed destination threshold,
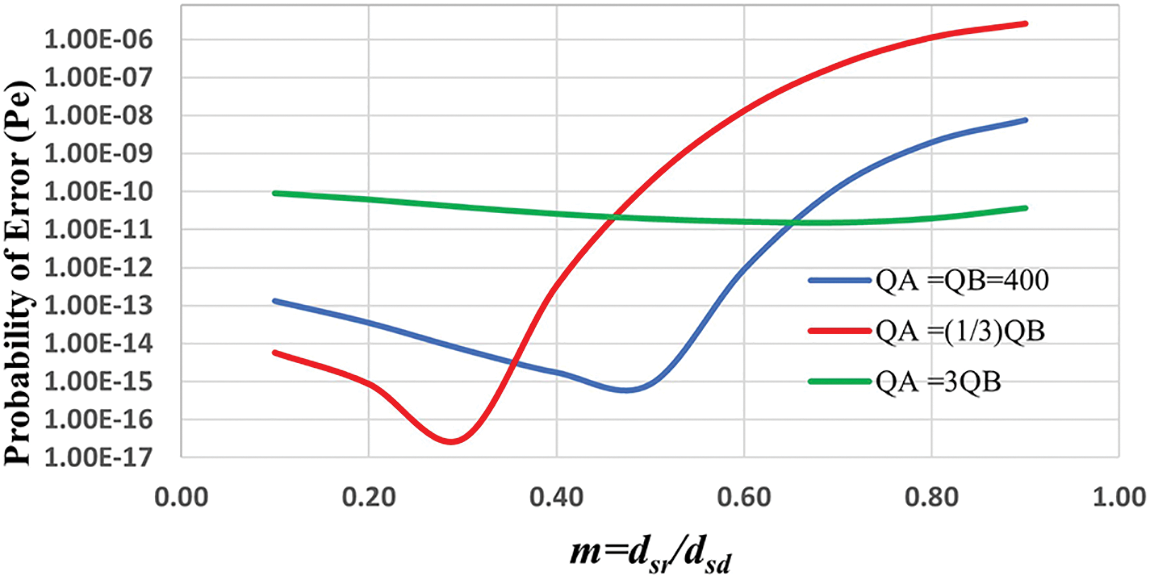
Figure 5: Probability of error vs. relay node position, when the detection threshold and velocity are fixed, (
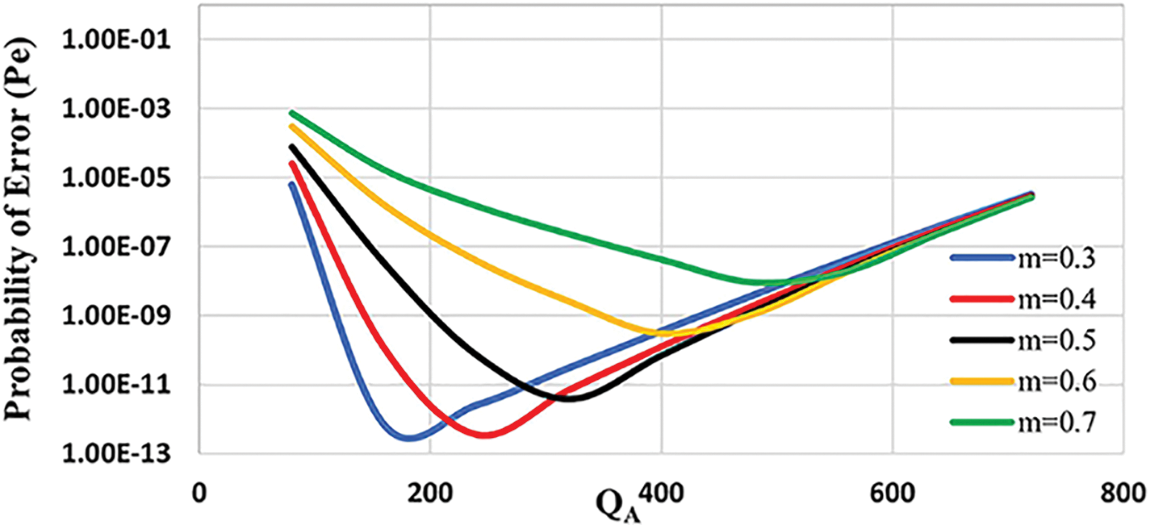
Figure 6: Probability of error vs. number of molecules assigned to node S for various relay node positions when the detection threshold and velocity are fixed (
Unlike the previous literature, the proposed nano-system is considered non-linear due to the effect of velocity that controls the diffusion of molecules and different types of molecules A and B with different diffusion coefficients, and thus the optimal relay position is obtained when
We can conclude from the preceding analysis that the proposed cooperative nano-system with nano-relay outperforms the DLR system. Furthermore, when compared to the DLR system, the proposed nano-system uses a lower molecular budget with low error probability.
Table 3 illustrates a comparison between PSO and MCFO before and after optimizing the flow velocity

This study proposes a nano-system with cooperative molecular communications based on nano-relaying. It considers the molecular drift velocity in the proposed molecular communication system based on diffusion. The noise and ISI issues have an impact on the molecular diffusion channel. Furthermore, we proposed a collaborative optimization problem for nano relay positioning and determination of the fraction of the drug molecular budget that should be allocated to the nano transmitter and nano receiver. The probability of bit error is expressed in closed form, and this is used as an objective function to determine the optimal velocity of the drug molecules and the detection threshold at the nano receiver. The error probability equation is then used as an objective function to determine the optimal drug molecule velocity and detection threshold to achieve a minimum probability oferror. The numerical results show that the proposed scheme can improve the performance of the nanosystem, when compared to a system with a direct link relay (DLR). Furthermore, this approach can be extended to a multi hop nano relay to make it more realistic for nano receivers with a large number of receptors. Finally, the proposed nano system with the cooperative nano relaying approach combined with the emerging nanotechnology can be implemented in advanced nanomedicine solutions to perform disease detection, health monitoring, and targeted drug delivery more efficiently.
Acknowledgement: None.
Funding Statement: This work was funded by the Researchers Supporting Project Number (RSP2023R102) King Saud University, Riyadh, Saudi Arabia.
Author Contributions: Saied M. Abd El-atty and Eman S. Attia conceived of the main idea, developed the theory, and performed the computations for the presented work. The analytical methods were validated by Ashraf A. M. Khalaf, Fathi E. Abd El-Samie, and Heba M. El-Hosen. The manuscript was written by Saied M. Abd El-atty, Eman S. Attia, Konstantinos A. Lizos, and Osama Alfarraj. The findings were considered by all writers, and they all contributed to the final publication. All authors discussed the results and contributed to the final manuscript.
Availability of Data and Materials: The authors have not used datasets generated during the paper work but the analysis and codes are available from the corresponding author on reasonable request.
Conflicts of Interest: The authors have no relevant financial or non-financial interests to disclose.
References
1. Abd El-atty, S. M. (2020). Health monitoring scheme based forster resonance energy transfer nanocommunications in the Internet of Biological Nanothings. International Journal of Communication Systems, 33(11), e4398. [Google Scholar]
2. Mohamed, S., Dong, J., El-Atty, S. M. A., Eissa, M. A. (2022). Bio-cyber interface parameter estimation with neural network for the Internet of Bio-Nano Things. Wireless Personal Communications, 123(2), 1245–1263. [Google Scholar]
3. El-Fatyany, A., Wang, H., El-atty, A., Saied, M. (2021). Efficient framework analysis for targeted drug delivery based on Internet of Bio-Nanothings. Arabian Journal for Science and Engineering, 46(10), 9965–9980. [Google Scholar] [PubMed]
4. Attia, E. S., Khalaf, A. A. M., Abd El-Samie, F. E., Abd El-Atty, S. M., Lizos, K. A. et al. (2022). Embedded coded relay system for molecular communications. Computers, Materials & Continua, 72, 2729–2748. [Google Scholar]
5. Chude-Okonkwo, U., Malekian, R., Maharaj, B. T. (2020). Advanced targeted nanomedicine a communication engineering solution, Cham: Springer. [Google Scholar]
6. Zafar, S., Nazir, M., Bakhshi, T., Khattak, H. A., Khan, S. et al. (2021). A systematic review of bio-cyber interface technologies and security issues for Internet of Bio-Nano Thing. IEEE Access, 9, 93529–93566. [Google Scholar]
7. Pan, W., Chen, X., Yang, X., Zhao, N., Meng, L. et al. (2022). A molecular communication platform based on Body Area Nanonetwork. Nanomaterials, 12(4), 722. [Google Scholar] [PubMed]
8. Abd El-atty, S. M., Arafa, N. A., Abouelazm, A., Alfarraj, O., Lizos, K. et al. (2022). Performance analysis of an artificial intelligence nanosystem with biological Internet of Nano Things. Computer Modeling in Engineering & Sciences, 133(1), 1–21. https://doi.org/10.32604/cmes.2022.020793 [Google Scholar] [PubMed] [CrossRef]
9. Farsad, N., Yilmaz, H. B., Eckford, A., Chae, C. B., Guo, W. (2016). A comprehensive survey of recent advancements in molecular communication. IEEE Communications Surveys Tutorials, 18(3), 1887–1919. [Google Scholar]
10. Kadloor, S., Adve, R. S., Eckford, A. W. (2012). Molecular communication using Brownian motion with drift. IEEE Transactions on NanoBioscience, 11(2), 89–99. [Google Scholar] [PubMed]
11. Gómez, J. T., Pitke, K., Stratmann, L., Dressler, F. (2022). Age of information in molecular communication channels. Digital Signal Processing, 124, 103108. [Google Scholar]
12. Kuscu, M., Akan, O. B. (2022). Detection in molecular communications with ligand receptors under molecular interference. Digital Signal Processing, 124, 103186. [Google Scholar]
13. He, S., Joseph, N., Feng, S., Jellicoe, M., Raston, C. L. (2020). Application of microfluidic technology in food processing. Food Function, 11(7), 5726–5737. [Google Scholar] [PubMed]
14. Yang, N. J., Chiu, I. M. (2017). Bacterial signaling to the nervous system through toxins and metabolites. Journal of Molecular Biology, 429(5), 587–605. https://doi.org/10.1016/j.jmb.2016.12.023 [Google Scholar] [PubMed] [CrossRef]
15. Cheng, Z., Sun, J., Yan, J., Tu, Y. (2022). Optimizations for mobile MIMO relay molecular communication via diffusion with network coding. KSII Transactions on Internet and Information Systems, 16(4), 1373–1391. [Google Scholar]
16. Akdeniz, B. C., Tepekule, B., Pusane, A. E., TuÄcu, T. (2017). Novel network coding approaches for diffusionabased molecular nanonetworks. Transactions on Emerging Telecommunications Technologies, 28(7), e3105. [Google Scholar]
17. Varshney, N., Haselmayr, W., Guo, W. (2018). On flow-induced diffusive mobile molecular communication: First hitting time and performance analysis. IEEE Transactions on Molecular, Biological and Multi-Scale Communications, 4(4), 195–207. [Google Scholar]
18. Tiwari, S. K., Reddy, T. R. T., Upadhyay, P. K., Da Costa, D. B. (2018). Joint optimization of molecular resource allocation and relay positioning in diffusive nanonetworks. IEEE Access, 6, 67681–67687. [Google Scholar]
19. Angjo, J., Pusane, A. E., Yilmaz, H. B., Basar, E., Tugcu, T. (2022). Optimal relaying in molecular communications. Nano Communication Networks, 32–33, 100404. [Google Scholar]
20. Carton, F., Malatesta, M. (2022). In vitro models of biological barriers for nanomedical research. International Journal of Molecular Sciences, 23(16), 8910. [Google Scholar] [PubMed]
21. Bozzuto, G., Molinari, A. (2015). Liposomes as nanomedical devices. International Journal of Nanomedicine, 10, 975–999. [Google Scholar] [PubMed]
22. Nuwairan, M. A., Chaabelasri, E. (2023). Numerical assessment of nanofluid natural convection using local RBF method coupled with an artificial compressibility model. Computer Modeling in Engineering & Sciences, 135(1), 133–154. https://doi.org/10.32604/cmes.2022.022649 [Google Scholar] [PubMed] [CrossRef]
23. Javaid, M., Alamer, A., Sattar, A. (2023). Topological aspects of dendrimers via connection-based descriptors. Computer Modeling in Engineering & Sciences, 135(2), 1649–1667. https://doi.org/10.32604/cmes.2022.022832 [Google Scholar] [PubMed] [CrossRef]
24. Mahmoud, K. R. (2016). Synthesis of unequally-spaced linear array using modified central force optimisation algorithm. IET Microwaves, Antennas Propagation, 10(10), 1011–1021. [Google Scholar]
25. Siddique, N., Adeli, H. (2015). Central force metaheuristic optimisation. Scientia Iranica, 22(6), 1941–1953. [Google Scholar]
26. Chen, Y., Yu, J., Mei, Y., Wang, Y., Su, X. (2016). Modified central force optimization (MCFO) algorithm for 3D UAV path planning. Neurocomputing, 171(3), 878–888. [Google Scholar]
27. Wang, X., Jia, Z. (2020). Reliability analysis of molecular communication based on drift diffusion in different topologies. Journal of Computer and Communications, 8(1), 71–89. [Google Scholar]
28. Tavakkoli, N., Azmi, P., Mokari, N. (2017). Optimal positioning of relay node in cooperative molecular communication networks. IEEE Transactions on Communications, 65(12), 5293–5304. [Google Scholar]
29. Tavakkoli, N., Azmi, P., Mokari, N. (2016). Performance evaluation and optimal detection of relay-assisted diffusion-based molecular communication with drift. IEEE Transactions on NanoBioscience, 16(1), 34–42. [Google Scholar] [PubMed]
30. Pierobon, M., Akyildiz, I. F. (2011). Diffusion-based noise analysis for molecular communication in nanonetworks. IEEE Transactions on Signal Processing, 59(6), 2532–2547. [Google Scholar]
31. Jamshidi, A., Keshavarz-Haddad, A., Ardeshiri, G. (2019). MAP detector performance analysis in diffusion-based relaying molecular communications. Nano Communication Networks, 19(12), 81–91. [Google Scholar]
32. Wang, J., Peng, M., Liu, Y., Liu, X., Daneshmand, M. (2019). Performance analysis of signal detection for amplify-and-forward relay in diffusion-based molecular communication systems. IEEE Internet of Things Journal, 7(2), 1401–1412. [Google Scholar]
Appendix
The steps for calculating the detection threshold at node R
The threshold at node R has a closed-form expression and can be determined carefully [28]. The likelihood-ratio test
Because the number of molecules in the medium cannot be negative, the negative value should be removed. Furthermore, because the number of molecules is an integer, the final positive answer must be rounded to an integer.
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools