 Open Access
Open Access
ARTICLE
Construction of a Computational Scheme for the Fuzzy HIV/AIDS Epidemic Model with a Nonlinear Saturated Incidence Rate
1 Department of Mathematics and Sciences, College of Humanities and Sciences, Prince Sultan University, Riyadh, 11586, Saudi Arabia
2 Department of Mathematics, Air University, Islamabad, 44000, Pakistan
* Corresponding Author: Muhammad Shoaib Arif. Email:
(This article belongs to the Special Issue: Recent Developments on Computational Biology-I)
Computer Modeling in Engineering & Sciences 2024, 138(2), 1405-1425. https://doi.org/10.32604/cmes.2023.028946
Received 18 January 2023; Accepted 18 July 2023; Issue published 17 November 2023
Abstract
This work aimed to construct an epidemic model with fuzzy parameters. Since the classical epidemic model does not elaborate on the successful interaction of susceptible and infective people, the constructed fuzzy epidemic model discusses the more detailed versions of the interactions between infective and susceptible people. The next-generation matrix approach is employed to find the reproduction number of a deterministic model. The sensitivity analysis and local stability analysis of the system are also provided. For solving the fuzzy epidemic model, a numerical scheme is constructed which consists of three time levels. The numerical scheme has an advantage over the existing forward Euler scheme for determining the conditions of getting the positive solution. The established scheme also has an advantage over existing non-standard finite difference methods in terms of order of accuracy. The stability of the scheme for the considered fuzzy model is also provided. From the plotted results, it can be observed that susceptible people decay by rising interaction parameters.Keywords
Epidemiology is the branch that offers predictions against infectious diseases, their presence, and control among a given population. It explains the factors governing infection spread and provides a suitable precautionary measure adapted for its control. Therefore, one can say that epidemiology is the branch that improves the healthcare system by regulating disease spread [1]. In 1981, the USA was the first country to report early cases of HIV (a retrovirus) among transgender people, plotting it as a life-threatening ailment. To date, 25 million deaths have been reported, with 14,000 new cases/day forming a nightmare with no cure as no vaccine has yet been prepared. It requires 6 months to 15 years for an individual to become completely susceptible to HIV and reveal the symptoms.
On the other hand, the virus severely disrupts the CD4+ T-cells, affecting the autoimmunity system of cells. Ultimately, individuals lacking immunity became vulnerable to even minute diseases like influenza, leading them straight to death. The routes for HIV movement are unprotected sexual intercourse along with contaminated syringes, needles, and blood, which may infect the person when in use. Blood containing HIV, when transferred through vertical transmissions [2], could be fatal for a receiver.
Mathematics has given researchers enormous tools to build the epidemiology of HIV/AIDS and understand the involvement of variable factors contributing to the epidemic. Three approaches to the population-based study of the HIV/AIDS epidemic were made by Zafar et al. [3]. The Adams-Bashforth Moulton method, Grunwald Letnikov approach, and Grunwald Letnikov approach with binomial coefficients. In this study, the stability if
A fuzzy differential equation is commonly used to omit uncertainty modeling a dynamic system. Such a system may involve cell growth and the dynamic of population, tumor growth, and nuclear disintegration under uncertainty. The transition of HIV to AIDS can be modeled using a fuzzy transference rate correlated with the viral load and CD4+ T-cells level by rule bases [5]. Several drugs have been designed that are optimal for drug therapy and prescribed to HIV patients. Considering the drug therapy, several questions have been asked about the best medicinal therapy for the HIV patient, although the therapeutic period is confined. The answer lies in the traditional differential equation, which efficiently describes the interaction between HIV and an infective individual’s immune system. This equation provides an appropriate optimal control on the system of equations by a suitable medicine program [6].
Individuals carrying HIV depicted variable levels of their immune system regarding the number of immunity cells vs. concentration of virus inside the body during a virus attack. This could be modeled using mathematical modeling that interprets the ratio between HIV and immune cells. Perelson et al. in [7] elaborated on three state variables, i.e., healthy CD4+ T-cells, infected CD4+ T-cells, and viruses, and created the basis of a simple mathematical model for HIV. Complexity arises when considering other variables like cytotoxic T lymphocytes and macrophages, as reported by [8]. Ambiguity in the given publication arises due to an unclarified uncertainty description without interpreting fuzzy parameters. The major concern of this paper is to focus on uncertain parameters and halt them using the fuzzy equation in preparation for the HIV model.
Hukuhara derivative [9] was the pioneer who suggested the concept of differentiability for fuzzy mappings about the fuzzy differential equation. Time revealed that the fuzzy differential equation used by the Hukuhara derivative could be termed fuzzier and possesses different characters compared to a crisp solution [10]. To remove this obstacle, another strong variable, fuzzy-number-valued functions, was derived, which exclusively generalized differentiability as mentioned in [11–14].
The simplest are first-order fuzzy differential equations that as many applications. Whereas such simplest equation creates hurdles under different fuzzy differential equation concepts, i.e., the behavior of solutions is different (based on interpretation). Several researchers have utilized first-order fuzzy linear equations, as mentioned in [11]. Under the umbrella of the generalized differentiability notion, the author proposed a general form of the solutions to the first-order fuzzy differential equations with crisp coefficients. Recently operator approach has been proposed for solving first- and second-order linear fuzzy differential equations under strongly generalized differentiability [12]. Boundary value conditions have been introduced to the impulses [13]. A first-order linear fuzzy differential equation’s explicit solutions are found by calculating them on each level set and proving their existence and uniqueness. They are using the generalized Euler approximation approach. The numerical solution of the fuzzy differential equation under generalized differentiability has been studied in [14]. A generalization of Hukuhara’s differentiability for interval-valued functions has been introduced in [15]. Under this sort of differentiability, interval differential equations have local existence and uniqueness.
Linear fuzzy differential dynamical systems with complex number representation of level sets and solutions derived under this representation were the focus of [16], which focused on linear fuzzy differential dynamical systems with initial conditions defined by a fuzzy number vector. When solving linear differential dynamical systems, including fuzzy matrices, it is helpful to keep this in mind, but [17] goes one step further by providing solutions to these systems using fuzzy matrices in addition to those provided by [18].
This publication provided sufficient information for the existence of equilibrium and global stability concerning the endemic and infection-free medium by formulating suitable Lyapunov functions. Fractional SEIR epidemic models and fractional differential equations were examined by Almeida [19] in an attempt to understand the dynamics of specific epidemics. Carvalho et al. developed a fractional-order model of HIV/HCV coinfection to understand better how the HIV viral load affects the coinfection [20]. Their study was confined to providing a numeric of individuals suffering from various infections like HIV, HCV, dengue fever, and many others. However, results revealed that the HIV viral load impacts remarkably on the harshness of the HCV infection, and the treatment efficacy is dominated by the natural progression of HCV against HIV/HCV coinfection. Using a multi-patch HIV/AIDS epidemic model with fractional-order derivatives, Kheiri et al. [21] studied the effect of human drive on HIV/AIDS pandemic propagation among patches. The major objective of the study was to bring the basic reproduction number
The model formulated by [21] comprised time-dependent control to minimize the spread of HIV/AIDS and the derived fractional optimal control that varies the fractional order of the disease spread. Nowadays, plenty of literature on fractional-order models of HIV disease dynamics reveals the engagement of mathematical techniques. Such techniques solve the models and the disease dynamics (HIV) spread [22,23]. Studies focused on fractional-order modeling can be seen in the literature [24–26] and references therein. The most significant application of fractional order derivative is modeling infectious disease, vibration equations [27,28], and much more.
Moreover, massive optimal control theory applications have made it applicable in several fields of pure and applied sciences [29]. The theory of linear systems has been extensively worked on [30]. Contrary, the theory of nonlinear optimal control (OCP) needed a thorough description and further analysis [31,32]. The modal series method and eigenvalue decomposition technique were used by Jajarmi et al. [33] to illustrate a class of nonlinear optimal control problems and convergence analysis. Jajarmi et al. [34] investigated the optimal control of time-varying delay systems with persistent matched external disturbances. The uniqueness of their study, which made the whole working thing interesting, is the conversion of the disturbed original time-delay model into an augmented system without any disturbance. A quadratic performance index was chosen for the enhanced system to establish an undisturbed time-delay optimum control issue. Two-point boundary value problems drive to advance and delay optimality criteria. An iterative algorithm for solving the latter advance-delay boundary value problem with a new iterative technique was also offered. To approximate the solution of the HIV infection of
This study concerns a fuzzy epidemic model comprising five compartments: Susceptible, exposed, unaware, infective, aware, and recovered. The fuzzy-based interaction parameters are defined and further used in the model. The fuzzy parameters are split into three parts that discuss the unsuccessful, successful, and fully successful meetings of infective with susceptible. If the value of the fuzzy parameter falls between 0 and 1, then a higher value corresponds to higher chances of successful interaction of infective with susceptible. So, two fuzzy parameters in a model provide nine different cases because each fuzzy parameter is divided into three parts. The discussions related to three cases exist in this study, but the remaining cases can be discussed similarly. In addition, a numerical scheme is established for three of the discussed cases. The numerical scheme has the advantage over some existing classical schemes in determining conditions for a positive solution. The numerical scheme is established on three time levels, so either a different scheme is adopted, or some initial guess is chosen for the second time level. In this work, the forward Euler scheme is chosen for finding a solution at the second time level. Since the given scheme and existing forward Euler schemes are conditionally stable, both require suitable step sizes for obtaining a convergent solution. Among existing finite difference schemes for solving differential equations, a non-standard finite difference method has been chosen by various researchers for solving epidemic models. The non-standard finite difference scheme has been constructed on two-time levels. It is unconditionally stable and provides surety to get positive solutions for some of the existing epidemic models. But, the scheme has its drawback: it does not achieve first-order accuracy in the obtained solution. So, the solution obtained by a non-standard scheme is doubtful unless the proper step size is chosen. The scheme constructed in this work has the advantage over the non-standard scheme due to the order of accuracy. In addition, the constructed scheme has an advantage over the forward Euler method in terms of enlarged stability region, but the constructed scheme is conditionally stable.
Before starting the mathematical modeling and analysis of the fuzzy epidemic model, some basic definitions are given.
2.1 Fuzzy Subset [40]
A fuzzy subset of a Universe
A fuzzy number is a generalization of a regular real number. It refers to a connected set of potential values instead of just one specific value, where each potential value weights 0 and 1. The membership function is the name of this weight. Consequently, a convex, normalized fuzzy set of the real line is a particular instance of a fuzzy number.
2.3 Triangular Fuzzy Number [40]
The number
where
2.4 Trapezoidal Fuzzy Number [40]
The number
where
2.5 Expected Value [41]
The expected value of a triangular fuzzy number
where
2.6 Fuzzy Basic Reproductive Number [41]
The fuzzy basic reproductive number of a triangular fuzzy number
The deterministic model given in [42] is expressed as:
where
Now, a fuzzy mathematical model with the incidence rate of AIDS is represented as:
where
The
The equilibrium point of the model (10)–(13) will be found in this section.
3.1 Fuzzy Equilibrium Analysis
The system (10)–(13) has disease-free equilibrium points
3.2 Fuzzy Basic Reproductive Number
The first basic reproductive number of the deterministic model will be found. For doing so, the next-generation matrix approach is considered. According to this approach, the following matrices can be found:
where
and
Since infected compartments are
The spectral radius of
The fuzzy basic reproductive number is given by:
Since there are three parts for each
Case I: In this case
Case II: For this case, let
The fuzzy basic reproductive number is given by:
Case III: When
where
Given the definition of the expected value,
For a parameter
The sensitivity of parameters is given by:
According to sensitivity analysis,
If
Proof: For proving this theorem, eigenvalues for the Jacobian system (10)–(13) must have non-positive eigenvalues. For doing so, consider the Jacobian of the system (10)–(13) in the form of
Case I: When
The eigenvalues of (35) are:
Since all eigenvalues are negative, system (10)–(13) is stable for this case.
Case II: In this case,
where
According to Routh Hurwitz’s criterion for cubic polynomials, the eigenvalues will lie in the left half plane, if and only if
The condition
All terms in (38) are positive except for three terms. Now, the second inequality in the theorem statement will be utilized.
Since
By using inequalities (39)–(41) in (38), it can be shown that
Case III: When
A numerical scheme is presented for solving systems (10)–(13). The scheme will be constructed for the model’s first Eq. (10). For doing so, consider the discretized equation for Eq. (10) as:
where
For finding
Substituting Taylor series expansions (43) and (44) into Eq. (42) yields
Eq. (45) can be expressed as:
Equating coefficients of
The unknown
Given Eqs. (48), (42) can be written as:
Similarly, the following equations can be obtained:
where
Case I: When
where
Case II: When
Case III: When
The rest of the equations will be the same as in this work.
For finding the stability conditions system of Eqs. (10)–(13) can be expressed as:
where
Discretizing Eq. (57) using the proposed scheme gives:
where
The characteristic polynomial for Eq. (59) can be expressed as:
The scheme will be stable if the following condition holds:
where
The fuzzy epidemic model has been constructed in this work. The model comprised fuzzy parameters that can be more generalized than classical ones. These given fuzzy parameters depend on the quantities of the interaction of people. The interaction parameters are divided into three categories. Suppose the quantities for the interaction of infective with susceptible are less than some minimum number. In that case, none of the susceptible will shift to the category of exposed people. If the interaction of people falls in a specific range, then the classical model can be used in which different values of parameters can be chosen. If the interaction of people is greater than some number, then there will be a 100% chance that the susceptible will become an exposed individual. So, nine possible cases can be discussed based on these three cases for one parameter. But, in this work, only a few cases have been discussed, and the rest can be discussed similarly. The presented numerical scheme has been constructed for three cases with specific values of
Fig. 1 shows the effect of

Figure 1: Effect of parameter

Figure 2: Effect of parameter

Figure 3: Effect of parameter

Figure 4: Effect of parameter

Figure 5: Effect of parameter

Figure 6: Effect of parameter

Figure 7: Effect of parameter
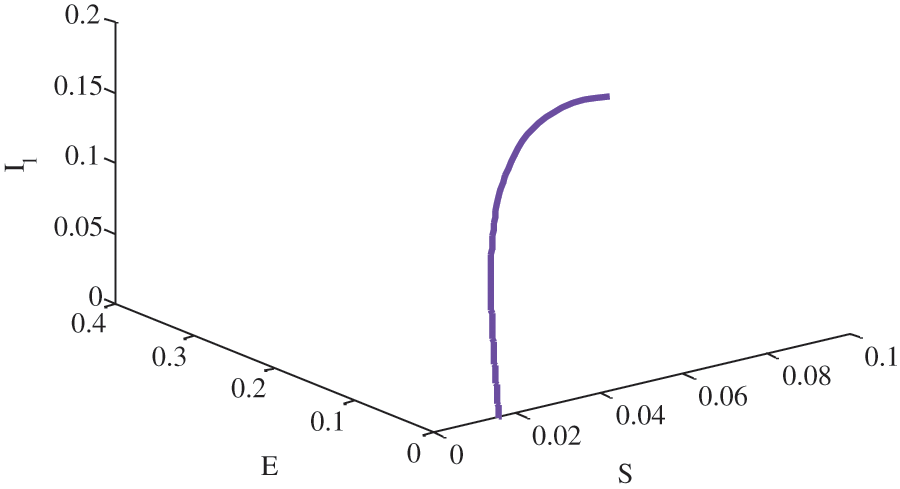
Figure 8: Relationship between susceptible, exposed and unaware infective people without birth rate of susceptible people using

Figure 9: Relationship between susceptible, exposed and unaware infective people with a birth rate of

Figure 10: Relationship between susceptible, exposed and unaware infective people with a birth rate of

Figure 11: Relationship between susceptible, exposed and unaware infective people with a birth rate of
Table 1 shows the comparison of schemes for finding errors. Since the exact solution of the considered system is not available, a numerical solution is adopted. The Matlab solver ode45 obtains the numerical solution. Looking at Table 1, it can be concluded that the proposed scheme performs better than the existing non-standard finite difference method in most cases.

A fuzzy epidemic model has been proposed in this work. The model consists of five compartments. The fuzzy transmission rates have been defined in the model. The fuzzy transmission rate parameters have been divided into three parts based on three possibilities for the interaction of unaware and aware infective with susceptible people. The numerical scheme has been established for some fuzzy parameter cases. The concluding points can be expressed as:
• The established scheme had an advantage over the existing forward Euler method in finding conditions for positive solutions.
• Also, the constructed scheme produced less error than the existing NSFD method in small step sizes.
• Susceptible people de-escalated, and exposed people escalated by incrementing fuzzy transmission parameters for unaware infective.
• Both types of infectives were de-escalated by growth in the AIDS parameter.
• Both types of infectives had dual behavior by raising transferring parameters from exposed to unaware infective.
• The plots for relationships between susceptible, exposed, and infective converged to the model's equilibrium points with the susceptible people’s birth rate.
The established numerical scheme in this work can be employed in different branches of science and engineering to solve similar types of fuzzy differential equations. The findings of this study may lead to novel uses for the present method. Different types of theoretical and applied fuzzy differential equations might be amenable to the proposed technique. It can be put into action with little effort. After this study is over, the current method could be used in a variety of contexts [43, 44].
Acknowledgement: The authors wish to express their gratitude to Prince Sultan University for facilitating the publication of this article through the Theoretical and Applied Sciences Lab.
Funding Statement: The authors would like to acknowledge the support of Prince Sultan University for paying the article processing charges (APC) of this publication.
Author Contributions: Conceptualization, methodology, and analysis, writing—review and editing, Y.N.; funding ac-quisition, K.A.; investigation, Y.N.; methodology, M.S.A.; project administration, K.A.; resources, K.A.; supervision, M.S.A.; visualization, K.A.; writing—review and editing, M.S.A.; proofreading and editing, M.S.A. All authors have read and agreed to the published version of the manuscript.
Availability of Data and Materials: The manuscript included all required data and implementing information.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Dubey, P., Dubey, U. S., Dubey, B. (2018). Modeling the role of acquired immune response and antiretroviral therapy in the dynamics of HIV infection. Mathematics and Computers in Simulation, 144(1450), 120–137. [Google Scholar]
2. Dubey, B., Dubey, P., Dubey, U. S. (2015). Dynamics of an SIR model with nonlinear incidence and treatment rate. Applications and Applied Mathematics: An International Journal (AAM), 10(2), 5. [Google Scholar]
3. Zafar, Z. U. A., Rehan, K., Mushtaq, M. (2017). HIV/AIDS epidemic fractional-order model. Journal of Difference Equations and Applications, 23(7), 1298–1315. [Google Scholar]
4. Wang, X., Wang, Z., Huang, X., Li, Y. (2018). Dynamic analysis of a delayed fractional-order SIR model with saturated incidence and treatment functions. International Journal of Bifurcation and Chaos, 28(14), 1850180. [Google Scholar]
5. Zarei, H., Kamyad, A. V., Heydari, A. A. (2012). Fuzzy modelling and control of HIV infection. Computational and Mathematical Methods in Medicine, 2012, 1–17. [Google Scholar]
6. Zadeh, H. G., Nejad, H. C. (2012). Presentation of a fast solution for solving HIV-infection dynamics and chemotherapy optimization based on fuzzy: AVK method. Journal of AIDS and HIV Research, 4(3), 60–67. [Google Scholar]
7. Perelson, A. S., Neumann, A. U., Markowitz, M., Leonard, J. M., Ho, D. D. (1996). HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science, 271(5255), 1582–1586. [Google Scholar] [PubMed]
8. Hadjiandreou, M. M., Conejeros, R., Wilson, D. I. (2009). Long-term HIV dynamics subject to continuous therapy and structured treatment interruptions. Chemical Engineering Science, 64(7), 1600–1617. [Google Scholar]
9. Kaleva, O. (1987). Fuzzy differential equations. Fuzzy Sets and Systems, 24(3), 301–317. [Google Scholar]
10. Diamond, P., Kloeden, P. (1994). Metric spaces of fuzzy sets. Singapore: World Scientific. [Google Scholar]
11. Khastan, A., Nieto, J. J., Rodriguez-Lopez, R. (2011). Variation of constant formula for first order fuzzy differential equations. Fuzzy Sets and Systems, 177(1), 20–33. [Google Scholar]
12. Allahviranloo, T., Abbasbandy, S., Salahshour, S., Hakimzadeh, A. (2011). A new method for solving fuzzy linear differential equations. Computing, 92(2), 181–197. [Google Scholar]
13. Nieto, J. J., Rodríguez-López, R., Villanueva-Pesqueira, M. (2011). Exact solution to the periodic boundary value problem for a first-order linear fuzzy differential equation with impulses. Fuzzy Optimization and Decision Making, 10(4), 323–339. [Google Scholar]
14. Nieto, J. J., Khastan, A., Ivaz, K. (2009). Numerical solution of fuzzy differential equations under generalized differentiability. Nonlinear Analysis: Hybrid Systems, 3(4), 700–707. [Google Scholar]
15. Stefanini, L., Bede, B. (2009). Generalized Hukuhara differentiability of interval-valued functions and interval differential equations. Nonlinear Analysis: Theory, Methods & Applications, 71(3–4), 1311–1328. [Google Scholar]
16. Pearson, D. W. (1997). A property of linear fuzzy differential equations. Applied Mathematics Letters, 10(3), 99–103. [Google Scholar]
17. Ghazanfari, B., Niazi, S., Ghazanfari, A. G. (2012). Linear matrix differential dynamical systems with fuzzy matrices. Applied Mathematical Modelling, 36(1), 348–356. [Google Scholar]
18. Xu, J., Liao, Z., Nieto, J. J. (2010). A class of linear differential dynamical systems with fuzzy matrices. Journal of Mathematical Analysis and Applications, 368(1), 54–68. [Google Scholar]
19. Almeida, R. (2018). Analysis of a fractional SEIR model with treatment. Applied Mathematics Letters, 84(4), 56–62. [Google Scholar]
20. Carvalho, A. R., Pinto, C., Baleanu, D. (2018). HIV/HCV coinfection model: A fractional-order perspective for the effect of the HIV viral load. Advances in Difference Equations, 2018(1), 1–22. [Google Scholar]
21. Kheiri, H., Jafari, M. (2019). Stability analysis of a fractional order model for the HIV/AIDS epidemic in a patchy environment. Journal of Computational and Applied Mathematics, 346(7181), 323–339. [Google Scholar]
22. Kermack, W. O., McKendrick, A. G. (1933). Contributions to the mathematical theory of epidemics. III.—Further studies of the problem of endemicity. Proceedings of the Royal Society of London, 141(843), 94–122. [Google Scholar]
23. Ding, Y., Ye, H. (2009). A fractional-order differential equation model of HIV infection of CD4+ T-cells. Mathematical and Computer Modelling, 50(3–4), 386–392. [Google Scholar]
24. Khan, T., Ullah, R., Abdeljawad, T., Alqudah, M. A., Faiz, F. (2023). A theoretical investigation of the SARS-CoV-2 model via fractional order epidemiological model. Computer Modeling in Engineering & Sciences, 135(2), 1295–1313. https://doi.org/10.32604/cmes.2022.022177 [Google Scholar] [PubMed] [CrossRef]
25. Shah, K., Abdeljawad, T., Jarad, F., Al-Mdallal, Q. (2023). On nonlinear conformable fractional order dynamical system via differential transform method. Computer Modeling in Engineering & Sciences, 136(2), 1457–1472. https://doi.org/10.32604/cmes.2023.021523 [Google Scholar] [PubMed] [CrossRef]
26. Shah, K., Naz, H., Abdeljawad, T., Abdalla, B. (2023). Study of fractional order dynamical system of viral infection disease under piecewise derivative. Computer Modeling in Engineering & Sciences, 136(1), 921–941. https://doi.org/10.32604/cmes.2023.025769 [Google Scholar] [PubMed] [CrossRef]
27. Kumar, D., Singh, J., Al Qurashi, M., Baleanu, D. (2019). A new fractional SIRS-SI malaria disease model with application of vaccines, antimalarial drugs, and spraying. Advances in Difference Equations, 2019(1), 1–19. [Google Scholar]
28. Kumar, D., Singh, J., Baleanu, D. (2020). On the analysis of vibration equation involving a fractional derivative with Mittag-Leffler law. Mathematical Methods in the Applied Sciences, 43(1), 443–457. [Google Scholar]
29. Geering, H. P. (2007). Optimal control with engineering applications, vol. 113. Berlin: Springer. [Google Scholar]
30. Jia, W., He, X., Guo, L. (2017). The optimal homotopy analysis method for solving linear optimal control problems. Applied Mathematical Modelling, 45, 865–880. [Google Scholar]
31. Lin, H., Wei, Q., Liu, D. (2017). Online identifier-actor-critic algorithm for optimal control of nonlinear systems. Optimal Control Applications and Methods, 38(3), 317–335. [Google Scholar]
32. Almobaied, M., Eksin, I., Guzelkaya, M. (2018). Inverse optimal controller based on extended Kalman filter for discrete-time nonlinear systems. Optimal Control Applications and Methods, 39(1), 19–34. [Google Scholar]
33. Jajarmi, A., Baleanu, D. (2018). Optimal control of nonlinear dynamical systems based on a new parallel eigenvalue decomposition approach. Optimal Control Applications and Methods, 39(2), 1071–1083. [Google Scholar]
34. Jajarmi, A., Hajipour, M., Baleanu, D. (2018). A new approach for the optimal control of time-varying delay systems with external persistent matched disturbances. Journal of Vibration and Control, 24(19), 4505–4512. [Google Scholar]
35. Attaullah, R. D., Weera, W. (2022). Galerkin time discretization scheme for the transmission dynamics of HIV infection with non-linear supply rate. Journal of AIMS Mathematics, 6(6), 11292–11310. [Google Scholar]
36. Alyobi, S., Yassen, M. F. (2022). A study on the transmission and dynamical behavior of an HIV/AIDS epidemic model with a cure rate. AIMS Mathematics, 7(9), 17507–17528. [Google Scholar]
37. Verma, L., Meher, R. (2022). Study on generalized fuzzy fractional human liver model with Atangana–Baleanu–Caputo fractional derivative. The European Physical Journal Plus, 137(11), 1–20. [Google Scholar]
38. Verma, L., Meher, R., Avazzadeh, Z., Nikan, O. (2022). Solution for generalized fuzzy fractional Kortewege-de Varies equation using a robust fuzzy double parametric approach. Journal of Ocean Engineering and Science, 3(7), 109. https://doi.org/10.1016/j.joes.2022.04.026 [Google Scholar] [CrossRef]
39. Tanriverdi, T., Baskonus, H. M., Mahmud, A. A., Muhamad, K. A. (2021). Explicit solution of fractional order atmosphere-soil-land plant carbon cycle system. Ecological Complexity, 48(4), 100966. [Google Scholar]
40. Barros, L. C. D., Bassanezi, R. C., Lodwick, W. A. (2017). A first course in fuzzy logic, fuzzy dynamical systems, and biomathematics: Theory and applications. Brazil: Springer. [Google Scholar]
41. Mangongo, Y. T., Bukweli, J. D. K., Kampempe, J. D. B. (2021). Fuzzy global stability analysis of the dynamics of malaria with fuzzy transmission and recovery rates. American Journal of Operations Research, 11(6), 257–282. [Google Scholar]
42. Naik, P. A., Zu, J., Owolabi, K. M. (2020). Global dynamics of a fractional order model for the transmission of HIV epidemic with optimal control. Chaos, Solitons & Fractals, 138(2), 109826. [Google Scholar]
43. Shatanawi, W., Raza, A., Arif, M. S., Rafiq, M., Bibi, M. et al. (2021). Essential features preserving dynamics of stochastic Dengue model. Computer Modeling in Engineering & Sciences, 126(1), 201–215. https://doi.org/10.32604/cmes.2021.012111 [Google Scholar] [PubMed] [CrossRef]
44. Abodayeh, K., Raza, A., Arif, M. S., Rafiq, M., Bibi, M. et al. (2020). Stochastic numerical analysis for impact of heavy alcohol consumption on transmission dynamics of gonorrhoea epidemic. Computers, Materials & Continua, 62(3), 1125–1142. https://doi.org/10.32604/cmc.2020.08885 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools