 Open Access
Open Access
ARTICLE
Deep Learning Approach for Automatic Cardiovascular Disease Prediction Employing ECG Signals
1
Department of Computer Science & Information Technology, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
2
Department of Computer Engineering, College of Computer Engineering and Sciences, Prince Sattam Bin Abdulaziz University,
Al-Kharj, 11942, Saudi Arabia
3
Department of Computer Science, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan,
Pakistan
4
Department of Cybersecurity, College of Computer Science and Engineering, University of Jeddah, Jeddah, 21959, Saudi Arabia
5
Faculty of Computer Science and Information Technology, Universiti Tun Husein Onn Malaysia (UTHM), Bahru, 80536, Malaysia
6
Research Centre, Future University in Egypt, New Cairo, 11745, Egypt
7
Department of Information Systems, University of Maryland, Baltimore County, Baltimore, MD 21250, USA
8
Department of Information and Communication Engineering, Yeungnam University, Gyeongsan-si, 38541, Korea
* Corresponding Authors: Imran Ashraf. Email: ;
(This article belongs to the Special Issue: Bio-inspired Computer Modelling: Theories and Applications in Engineering and Sciences)
Computer Modeling in Engineering & Sciences 2023, 137(2), 1677-1694. https://doi.org/10.32604/cmes.2023.026535
Received 10 September 2022; Accepted 08 February 2023; Issue published 26 June 2023
Abstract
Cardiovascular problems have become the predominant cause of death worldwide and a rise in the number of patients has been observed lately. Currently, electrocardiogram (ECG) data is analyzed by medical experts to determine the cardiac abnormality, which is time-consuming. In addition, the diagnosis requires experienced medical experts and is error-prone. However, automated identification of cardiovascular disease using ECGs is a challenging problem and state-of-the-art performance has been attained by complex deep learning architectures. This study proposes a simple multilayer perceptron (MLP) model for heart disease prediction to reduce computational complexity. ECG dataset containing averaged signals with window size 10 is used as an input. Several competing deep learning and machine learning models are used for comparison. K-fold cross-validation is used to validate the results. Experimental outcomes reveal that the MLP-based architecture can produce better outcomes than existing approaches with a 94.40% accuracy score. The findings of this study show that the proposed system achieves high performance indicating that it has the potential for deployment in a real-world, practical medical environment.Keywords
Cardiovascular diseases have become one of the biggest causes of death worldwide and a gradual rise has been seen in recent years. The World Health Organization (WHO) claims that the number of cardiovascular causing deaths reached 17.9 million and 80% of the deaths are related to coronary artery disease [1]. The death toll is high in low-income countries [2]. Heart diseases weaken the patient’s body, especially in adults, and damage the blood vessels [3]. Genetic factors and personal habits cause heart disease. Various other risk factors like smoking, overuse of alcohol, physical inactivity, high cholesterol, obesity, and hypertension are also attributed to heart disease. Thus, early detection of cardiovascular illness is crucial for prompt action to prevent death and other health complications.
Electrocardiogram (ECG) is a technique that is widely used in clinical practice by both cardiologists and non-cardiologists. The heart’s electrical activity is reflected in the ECG signal, and changes in its waveform or heart rhythm disturbances are indicators of underlying cardiovascular issues [4]. Even in environments with a lack of resources, the ECG is a quick, easy, and inexpensive diagnostic that is available. The test offers a window into the heart’s physiological and anatomical state, but it can also offer helpful diagnostic hints for disorders that affect the entire body. Although the ECG recording collection process is highly structured and reproducible, the interpretation of the ECG varies substantially depending on degrees of experience and skill. Computer-generated interpretations have been utilized for several years. These interpretations rely on feature recognition algorithms and predetermined criteria, which do not always account for the nuanced and intricate nature of an ECG [5].
Several different approaches can be used for cardiovascular disease that utilizes electrocardiogram (ECG) data. Disturbance in heart activity can be observed using ECG signals that record the electric activity of the heart [6]. Predominantly, heart abnormalities are identified by medical experts by visual analysis of ECG data; however, it requires experienced and specialized medical staff. Identifying abnormality in ECG waveform is a challenging task because visual identification takes time and effort. Even so, this method is time-consuming, error-prone, and subjective where the outcome depends on the observation and judgment of a medical expert. Consequently, automatic detection of cardiovascular diseases using artificial intelligent approaches and tools has become prevalent over the past few years. For example, a real-time ECG sensor-based automatic detection approach is designed in [7]. Similarly, a real-time portable ECG sensor is developed in [8]. Authors detected heart defects using ECG signals in [9]. Such approaches although rely on the ECG data, differ in the use of the ECG data for heart disease detection. Broadly, ECG can be used as an image for disease detection, or the numeric data recorded from the ECG sensor can be utilized. Image processing is carried out for the former category, which provides good results but can be computationally complex. Contrarily, the latter category takes advantage of ECG data properties along with personal traits of the patient like smoking habits, alcohol history, etc., for heart disease detection. Both categories have their pros and cons and have limitations regarding the provided accuracy. Techniques for feature selection are frequently used by researchers to increase the accuracy of results for high-dimensional data. But in many cases, models get overfit or underfit. Deep neural networks are commonly considered the best approach in disease diagnosis like Parkinson’s disease diagnosis [10], skin disease diagnosis [11], electrocardiographic diagnosis [12], and cardiovascular disease diagnosis and monitoring [13–15]. However, such models are computationally complex and for appropriate training require huge volumes of data.
Conventional machine learning classifiers like support vector machines, logistic regression, etc., require manual feature extraction, and choosing the appropriate features is also significant to obtain better results. Heart disease is predicted by several machine learning classifiers like K nearest neighbor, Naive Bayes, decision tree, and random forest. Both ECG-based image processing approaches and ECG data-based feature extraction approaches are well-studied for heart disease detection. However, using the ECG signal data is an under-explored research area and is focused upon in this research. This study makes the following contributions in this regard:
• The study utilizes the data from ECG for cardiovascular disease detection. Existing methods either employ image-based approaches or approaches that use features from the data. This study utilizes the averaged signals from the ECG using a window size of 10 which helps to reduce the computational complexity and improve the disease detection performance.
• A multilayer perceptron (MLP) is designed for heart disease prediction. It is a simple yet efficient and robust model and produces better results with high accuracy and reduced computational complexity.
• Experiments are carried out using several deep learning models including convolution neural network (CNN), recurrent neural network (RNN), and long short-term memory (LSTM) model. In addition, logistic regression (LR), extra tree classifier (ETC), stochastic gradient descent (SGD), random forest (RF), and Gaussian Naive Bayes (GNB) are also employed. Performance comparison is carried out with existing works that utilize feature-based prediction. K-fold cross-validation is used to validate the results.
The remainder of the paper is structured into four sections. Section 2 describes several important heart disease detection approaches. The details related to the dataset, proposed model, and deep neural network models are provided in Section 3. The results are described in Section 4 and discussion in Section 5. Section 6 presents the conclusion and suggested future work.
Health care is an important research field and medical diagnosis has emerged as an important research area in health care. Especially, with the rise of advanced machine learning algorithms, several novel tools and techniques for disease diagnosis have been designed [16]. Though cardiovascular disease detection is complex, recent advances in artificial intelligence tools and techniques have opened new ways to deal with its challenges [17]. Heart failure is one of the leading causes of death in developing countries and several approaches have been developed in this regard [18,19].
Khalil et al. [20] predicted heart disease using ECG signals. For this purpose, the authors adopt a convolutional neural network (CNN) and stationary wavelet transform. An end-to-end architecture is designed that learns from ECG signals and wavelet coefficients for predicting six types of heartbeats. Results show an accuracy of 99.57% with the proposed approach. The authors adopt the use of fast correlation-based feature selection for removing redundant features for predicting heart disease. Particle swarm optimization is used to enhance deep learning models. A machine-learning model for predicting heart disease was created by Li et al. [21]. The study uses various feature redundant feature removal approaches like relief, minimal redundancy maximal relevance, etc., with machine learning models. For feature extraction, a fast conditional mutual information-based approach is proposed. The study uses 13 variables from the Cleveland heart disease dataset. An accuracy of 92.37% is reported using the support vector machine model.
Several machine learning classifiers are evaluated using the Cleveland heart disease dataset in [22]. Of the used models, SVM is reported to have the highest accuracy using a linear kernel. Similarly, Acharya et al. [23,24] investigated J48, Naive Bayes, RF, MLP, and several other machine learning models for predicting heart disease. The use of multiple tools like Rapid miner, Orange, and Matlab is investigated by [25] for performance comparison of many machine learning models for heart disease prediction. Feature extraction from different types of datasets is another challenging task in medical data exploration. Different techniques have been used by researchers to extract valuable information from healthcare records [26–28]. Machine learning models have been applied in real-time for predicting heart disease [3]. In this research, features are extracted by two different approaches namely relief and univariate from the healthcare dataset. The proposed approach used word embedding for feature extraction and long short-term memory (LSTM) for prediction.
In another research work, echocardiography-based data is used to forecast heart patients’ mortality rate in hospitals [29]. Features are extracted manually and then the prediction is performed using deep learning models. A rule-based approach is proposed to automate feature extraction and classification tasks in [30]. Most medical data is unstructured and it is challenging to manage it using a rule-based strategy. The risk score of heart patients is calculated on unstructured medical data of patients [31]. The authors extracted features using text mining from unstructured data and predicted death risks for diabetic patients. Several researchers applied individual models while others have used a hybrid approach by combining two or more classifiers. The authors introduced the hybrid random forest linear model for heart disease prediction in [32] and suggested using a hybrid model to boost the performance of heart disease prediction utilizing different feature selection techniques. Similarly, an IoT-based heart patient monitoring approach has been proposed in [33]. Authors combined various data mining techniques with synthetic monitoring oversampling techniques (SMOTE) [34]. A qualitative review of these models has been presented in [35].
Feature selection techniques have also been explored for heart disease diagnosis. RF has been applied using multi-sensors for heart disease prediction [36]. Significant features are selected using correlation and feature fusion is applied for better results. The proposed fusion-based model achieved robust results with eight features; however, the selected features are not enough to represent all risk factors of the disease. Uncertainty of health-related data is handled by fuzzy logic and features are extracted using wavelet transformation to reduce the complexity [37]. Fast Fourier transformation is used as a recommender system for cardiovascular disease prediction [38]. A method for selecting features based on the preliminary collection and chaos firefly is designed for heart disease classification [39].
In traditional machine learning models, features are extracted heuristically. It depends upon human knowledge and expertise and can help in certain conditions. Mostly only shallow features are being considered by humans. The authors presented the drawbacks of machine learning models after comparing them with deep learning models using ECG signals [40]. Deep learning models are trained using automatic feature extractions and overcome the drawbacks of handcrafted features. The authors discussed recent advancements in deep-learning models used for remote heart monitoring [41]. A smart heart monitoring framework based on a deep learning model MLP is designed for prediction [42]. The authors designed a decision support system based on LSTM and SVM using various sensors including ECG sensors [43].
Other researchers used CNN model to classify ECG signals. Deep learning models are very useful in classifying ECG signals more quickly and efficiently. In particular, CNN, a complex structure made up of several deep layers and characteristics that are hidden, has been utilized to successfully identify heart problems in addition to many other medical diseases [44–46]. There are multiple stages of CNN, including the convolution stage where features are derived from the input signals. The authors presented a CNN-based time adaptive approach for cardiovascular disease detection using ECG [47].
Predominantly, existing approaches rely on manual feature extraction and the use of ECG signal data is a less explored area for heart disease prediction. Similarly, a few works adopt image processing-based approaches where the images of ECG data are used for disease prediction. This study explored the use of ECG signals for the same task and proposed an MLP for this purpose.
This section provides the details of the dataset used in this study and a brief description of the deep learning models for completeness. This study applies four deep learning models namely CNN, RNN, LSTM, and MLP. Similarly, LR, ETC, SGD, RF, and GNB are also implemented. It also describes the architecture of the proposed MLP and the flow of the methodology adopted for experiments. Fig. 1 depicts the methodology adopted for this study.
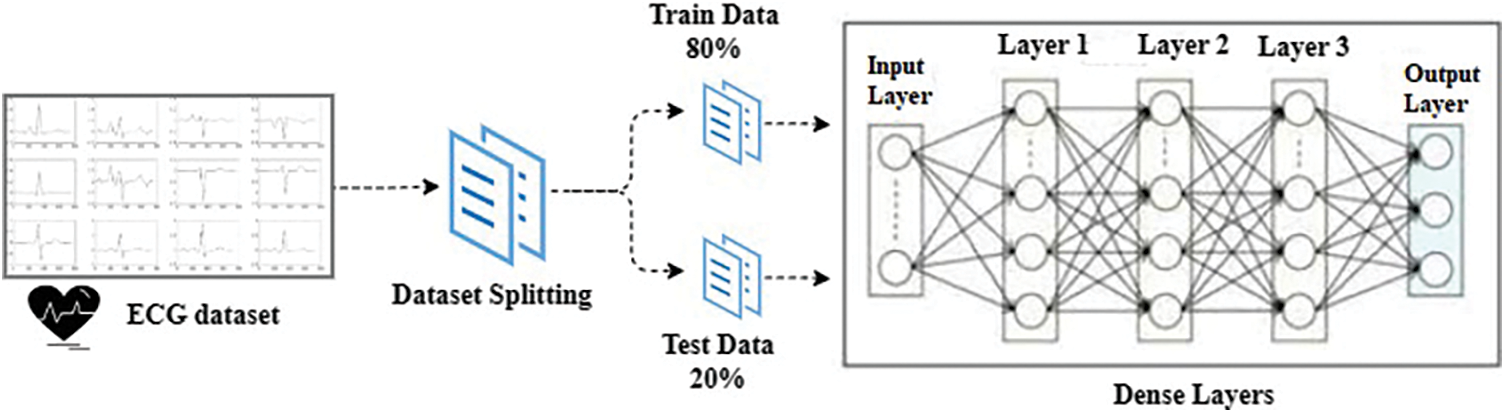
Figure 1: Architecture of the proposed methodology
The ECG dataset consists of electrocardiogram readings of patients. Each row represents the complete ECG reading of a patient [48]. Each ECG contains 140 readings. Columns 0–139 contain data points of a specific patient. The dataset is labeled categorically into two classes where the normal ECG is represented by ‘0’ and the abnormal ECG is represented by ‘1’. The ratio of heart patients is 41.60% while the normal people correspond to 58.40%. The samples for the abnormal and normal ECG are given in Fig. 2.
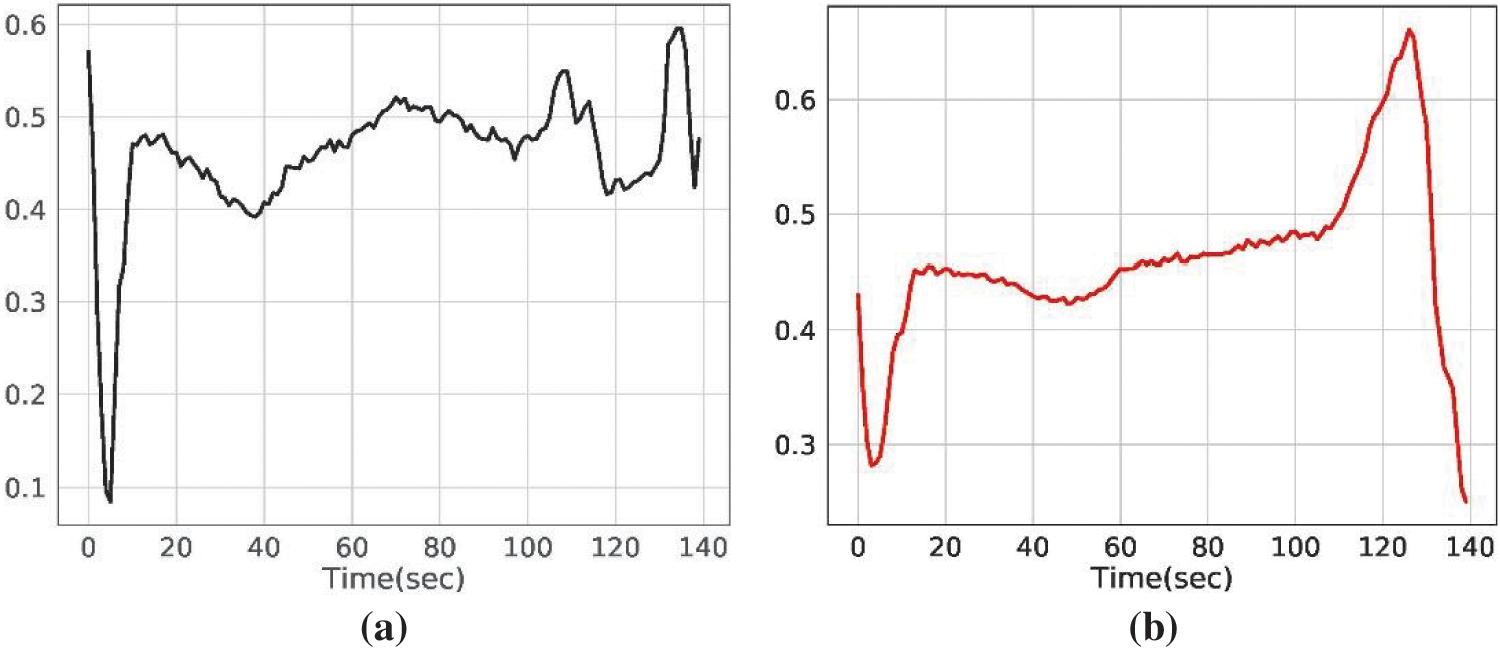
Figure 2: Electrocardiogram samples for (a) normal people, and (b) heart patients
Deep learning models have shown superb performance in several domains over the years including image classification [49,50], text analysis [51], disease detection [52,53], etc. Medical professionals have found automated systems to be very useful and successful tools for disease diagnosis. The deep neural network is a popular method for processing enormous amounts of data. Because it eliminates the need for manual feature extraction, it is currently widely employed in medical data analysis. Following is a brief explanation of the deep learning models that were deployed.
MLP has a number of distinguishing qualities concerning categorization, including being quick, simple to use and requires a small training set [54]. Input, output, and hidden layers make up the three primary layers of MLP. After processing, intermediary layers known as hidden layers join the input layer to the output layer. In MLP’s hidden layers, input values are multiplied by their corresponding weights,
where O stands for hidden layers and w denotes weight that is determined by gradient descent.
After determining the derivative of the error function, it updates some weights that were chosen at random to a negative gradient. Once an algorithm has gone through all possible iterations, training is stopped. The loss function is presented as:
where N presents the total number of training samples, qm presents the intended value and ym presents the desired value.
3.2.2 Convolutional Neural Network
Convolution, nonlinear activation, dropout, and pooling layers are key components of the effective neural network model CNN, which is used to learn complicated data [55]. It is intended for image-related tasks including image classification and segmentation. The end-to-end training process used by CNN makes it more effective. At the model’s end, fully connected layers are used to encode semantic information. It is a feed-forward network where features are mapped using filters that are applied to the output of the preceding layer. Convolutional layers extract features, and the output of the convolutional layers is then supplied to the fully connected layers. To reduce the size of feature maps, the pooling layer is a crucial step that is followed by a convolution layer [56]. The convolutional layers’ features are reduced by the Max-pooling to lessen overfitting. Max-pooling uses a max filter on (typically) non-overlapping subsections of the original representation. In Eq. (3), the max-pooling function is applied with size 9 to extract significant i features.
where i denotes the input and y is the activation output.
By putting weights on the kernel during the training phase, convolution layers extract local and high-level characteristics. CNN is frequently used to diagnose illnesses. Cross-entropy error is a loss function that has been employed in this study for binary classification. For binary classification, it is calculated as
If y stands for the class label indicator, p is the predicted probability, and the log is a natural log.
Sigmoid is an activation function in deep neural networks like CNN. Two neurons are produced for each instance of the target class by the CNN model. The output for the heart patient will be 1 for the first neuron and 0 for the other neurons. The values of the neurons will be reversed in a normal person.
For classification tasks in the medical field, CNN has been shown to be a reliable model. Many researchers have used CNN for different categorization tasks such as retinal disease classification [57], liver disease classification [58], malarial parasite detection [59], and brain tumor classification [60]. CNN has also been examined for text classification in earlier works, including hierarchical text classification [61] and clinical reports classification [62]. CNN attention module has been designed by researchers for COVID-19 diagnosis [63]. CNN has been used to identify myocardial infarction [62], atrial fibrillation [64], and abnormal arrhythmia detection [65] using ECG signals.
3.2.3 Recurrent Neural Network
The sequential deep neural network model is known as RNN [66]. During the processing of the following sequence, the state of the input sequence is retained. The sentence’s weighted data sequence is taken into account by RNN. By using a unique loop structure, it preserves previous knowledge. It is made to handle data, in particular, to anticipate the following data in a sentence.
With a more sophisticated RNN design, LSTM is more effective for long-term sequences [67]. LSTM overcomes the vanishing gradient problem that RNN encounters. It outperforms RNN by memorizing particular patterns. Three gates make up an LSTM: an input gate, an output gate, and a forget gate. Data sequences reported in Eqs. (5)–(7) are processed by LSTM.
where the input sequence is xt, the previous hidden state at step t is ht−1, the input gate is it, the output gate is ot, and the forget gate is ft.
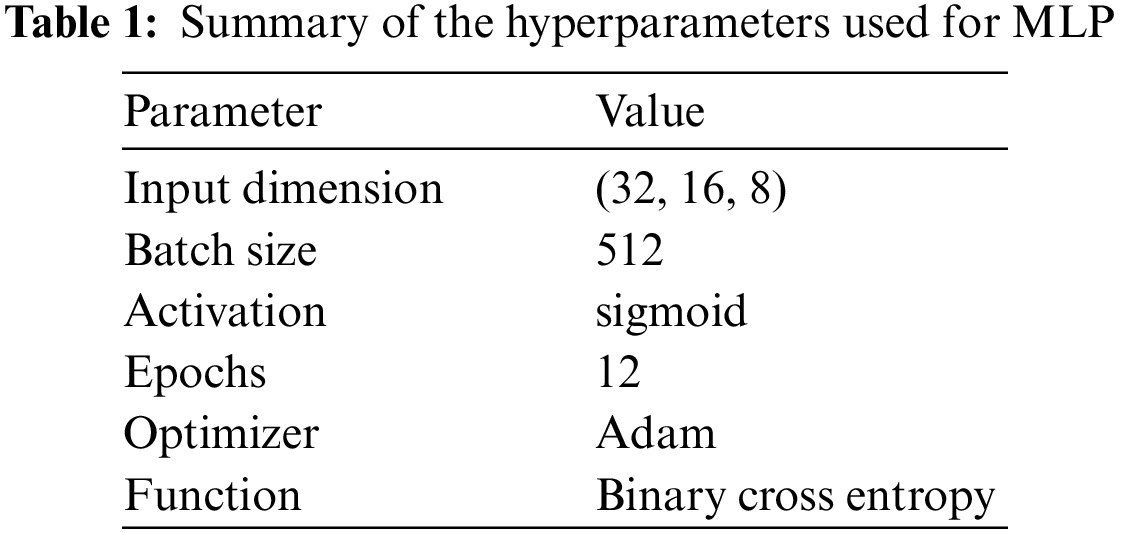
The ECG data is acquired for heart disease detection, followed by the train-test splitting for training and testing the deep neural network models in an 80:20 ratio. The proposed approach applied a simple three-layered MLP for the classification of ECG signals. Table 1 provides information about the model’s hyperparameters, activation, and optimization. Evaluation is based on accuracy, precision, recall, and F1 score, as well as k-fold cross-validation and performance comparison with existing studies.
Tensorflow and Keras libraries, which offer functions and routines to build neural network models as well as pre-trained models, are used in the experiments for the proposed study. The Python programming language and Anaconda platform are used to implement deep learning techniques. This research work makes use of a Dell Poweredge T430 server equipped with 32 GB of Random Access Memory (RAM), 8 intel Xeon cores of 3.4 GHz, 16 logical processors, and NVIDIA Quadro RTX 6000 24 GB Graphical Processing Unit (GPU). The data is divided into training and test sets at a ratio of 0 to 0.2 for heart disease prediction. In this case, four deep learning models MLP, CNN, RNN, and LSTM have been used. The train set consists of 3998 samples while the test set contains 1000 samples. Results from deep learning models have been contrasted with those from other models that have been documented.
4.1 Performance of Machine Learning Models
Experiments are carried out utilizing the chosen machine learning classifiers and the results are presented in Table 2. Experimental results show that SGD proves to be the best performer with a maximum 0.8988 accuracy while the precision, recall, and F1 score is 0.90 each. It is followed by the LR whose performance is marginally low with a 0.8902 accuracy score while the precision, recall, and F1 score is similar to SGD. NB model tends to show the lowest performance using the ECG data and obtains a 0.7661 accuracy value, as well as the lowest values for precision, recall, and F1 score. Results suggest that traditional machine learning models are not a good choice for ECG data-based heart disease detection as existing models already have similar or better results.
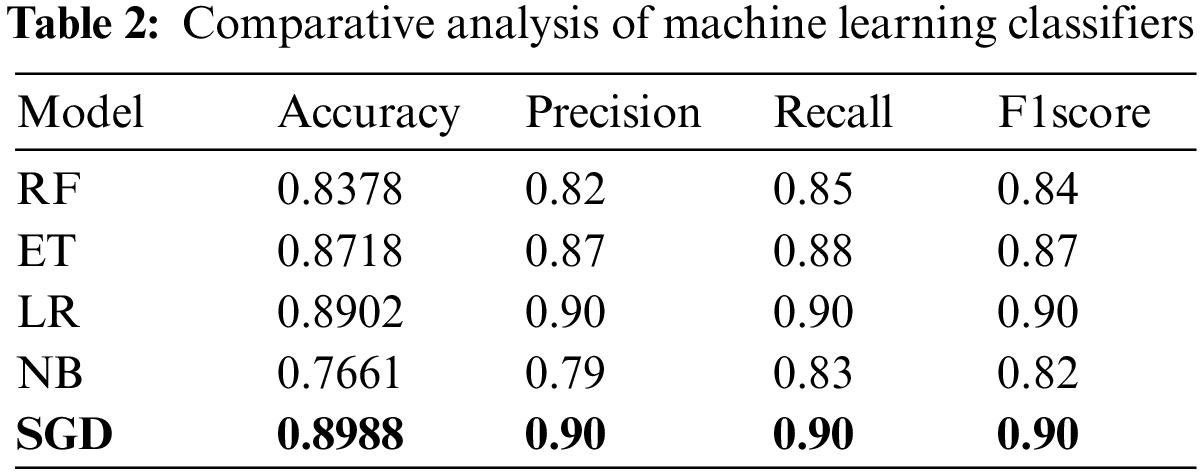
Fig. 3 shows the comparison of machine learning models for prediction in terms of accuracy, precision, recall, and F1 score. It indicates that the SGD classifier tends to show the best performance among machine learning models.
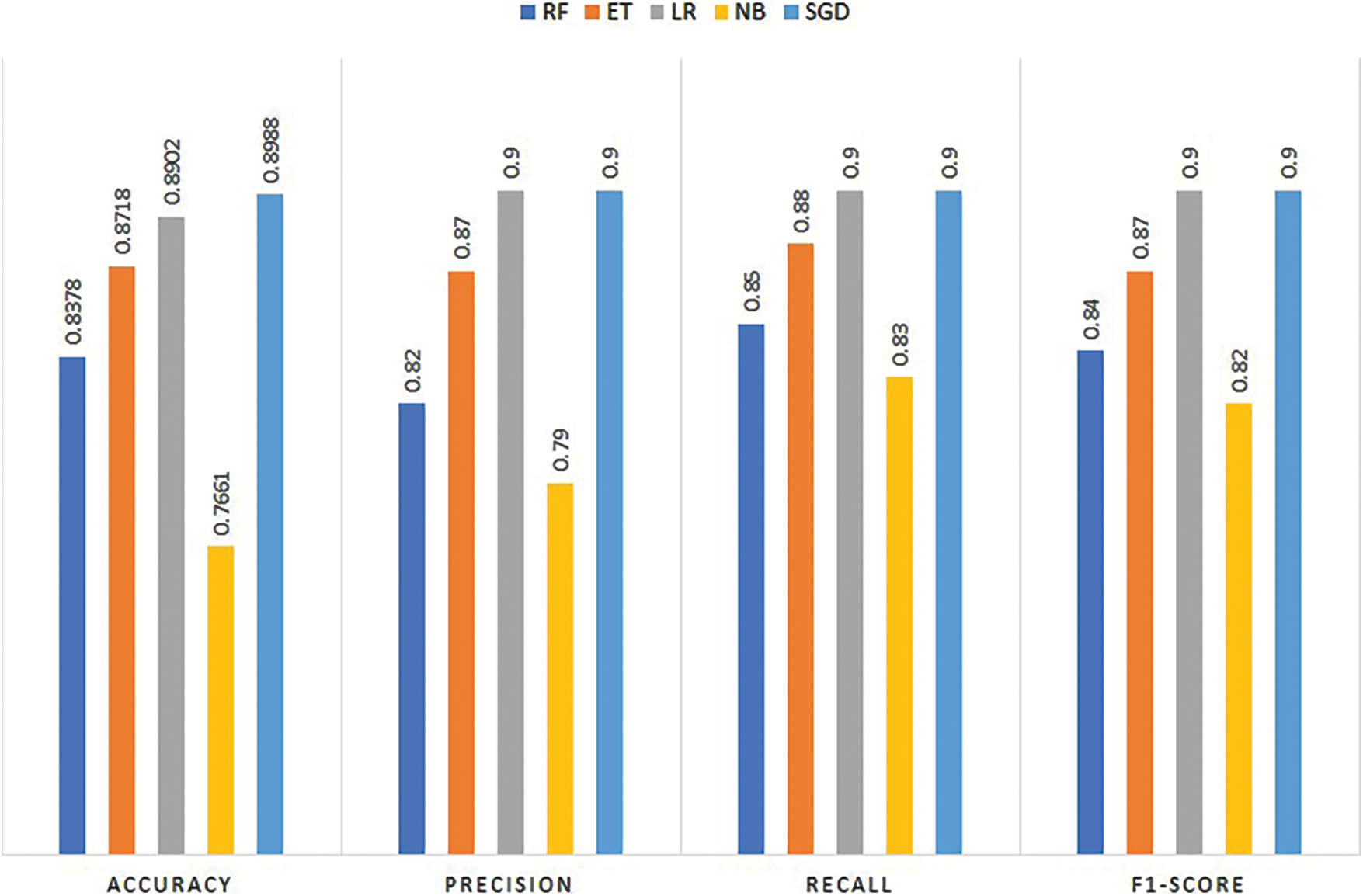
Figure 3: Performance comparison of machine learning models
4.2 Results of Deep Learning Models
Experiments have been performed by deploying deep neural network models using the ECG dataset. Table 3 presents the outcomes of the deep learning models. Results indicate that MLP shows the best scores with 0.9440 accuracy, 0.99 precision, 0.90 recall, and 0.94 F1 scores. CNN has also shown reasonable results with 0.9289 accuracy, 0.94 precision, 0.94 recall, and 0.94 F1 scores. The results from the LSTM model are slightly lower than CNN with a 0.9169 accuracy score and 0.92 scores for precision, recall, and F1 score. RNN shows the lowest performance of the model and it obtains 0.9001 accuracy, 0.88 precision, 0.90 recall, and 0.89 F1 score for heart disease prediction. Results indicate that the suggested MLP design performs better than other models.
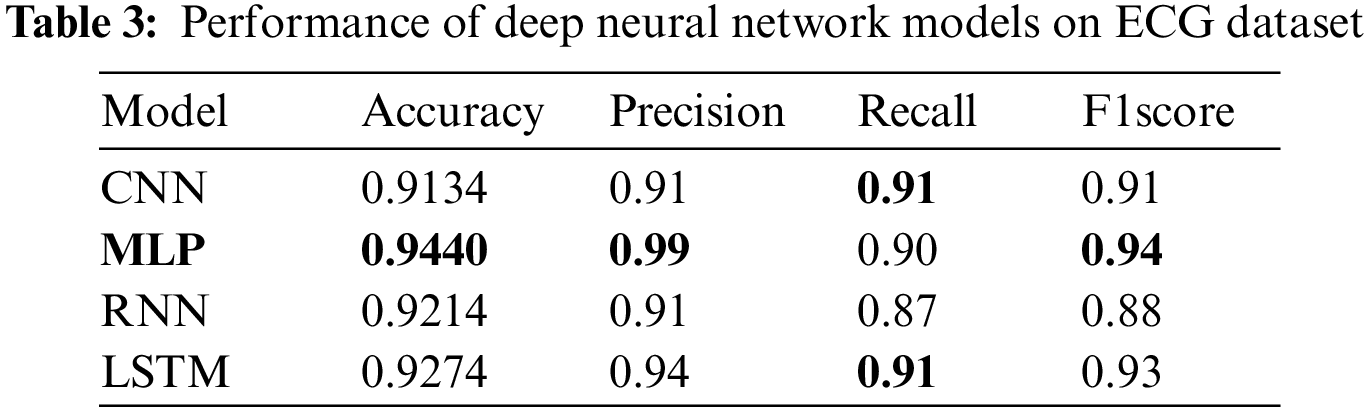
Despite the fact that MLP is not suitable for real-world computer vision tasks, for the current task where the ECG signals are used, its performance is far better than CNN, which proves to be the best for image processing tasks. For the heart disease detection task, the complexity of the MLP does not grow high and it produces robust results. CNN although a much better choice for image processing tasks, does not show better performance for ECG signal-based heart disease detection. Similarly, RNN works better for sequence prediction problems. However, training an RNN is difficult as compared to MLP and CNN, except for the LSTM. LSTM, which overcomes the limitation of the gradient vanishing point, is easy to train and shows better performance for a large range of problems. For heart disease prediction also, it shows the second-best performance with a 0.9274 accuracy. RNN and CNN show lower classification results for heart disease. MLP is suitable for predicting heart disease utilizing the ECG dataset and it predicts 508 heart patients correctly, and only 4 heart patients are misclassified.
Fig. 4 displays performance comparison results of deep learning models in terms of accuracy, precision, recall, and F1 score. Results reveal that the best performance is achieved by MLP.
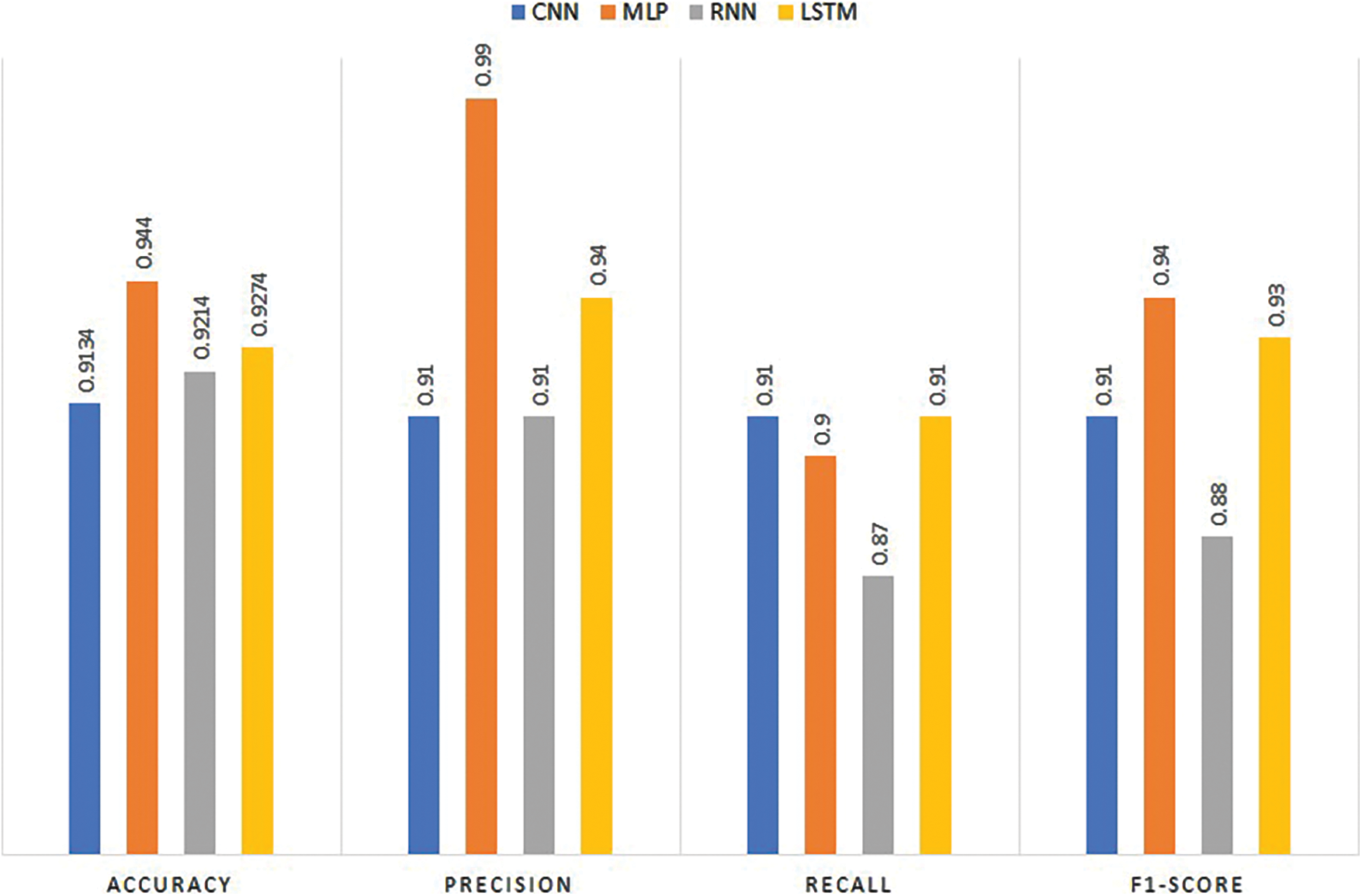
Figure 4: Performance of deep learning models
4.3 K-fold Cross-Validation Results
To confirm the effectiveness of the suggested approach, k-fold cross-validation is done using the pro- posed MLP model. Table 4 shows the scores attained by 10-fold cross-validation. Despite variations for each fold, the average accuracy of the MLP is 0.9408 for heart disease detection using ECG signals. Cross-validation results corroborate the effectiveness of the proposed MLP for heart disease detection.

Four deep-learning models (MLP, CNN, RNN, and LSTM) have been utilized in this study for heart disease prediction. Then comparative analysis was performed by analyzing their computational complexity and by comparing results with the state-of-the-art approaches from the literature.
5.1 Computational Complexity of Deep Neural Network Models
To analyze the applicability of the deep neural network models for real-time solutions, their computational complexity is evaluated by investigating the processing time for the ECG dataset. Results shown in Table 5 suggest that the proposed MLP proves to take less time for training and can provide robust results.

5.2 Comparison with Existing Methods
For performance comparison with existing approaches, we have selected two of our recent works on cardiovascular disease prediction where many machine learning models are applied using manual feature extraction. Reference [34] used DT, AdaBoost, LR, SGD, RF, GBM, ETC, GNB and SVM for heart disease prediction. Experiments are performed with and without SMOTE on all features and nine significant features. Results from [34] using selective features with SMOTE are the best and are added here for comparison. Table 6 presents the output of machine learning classifiers.
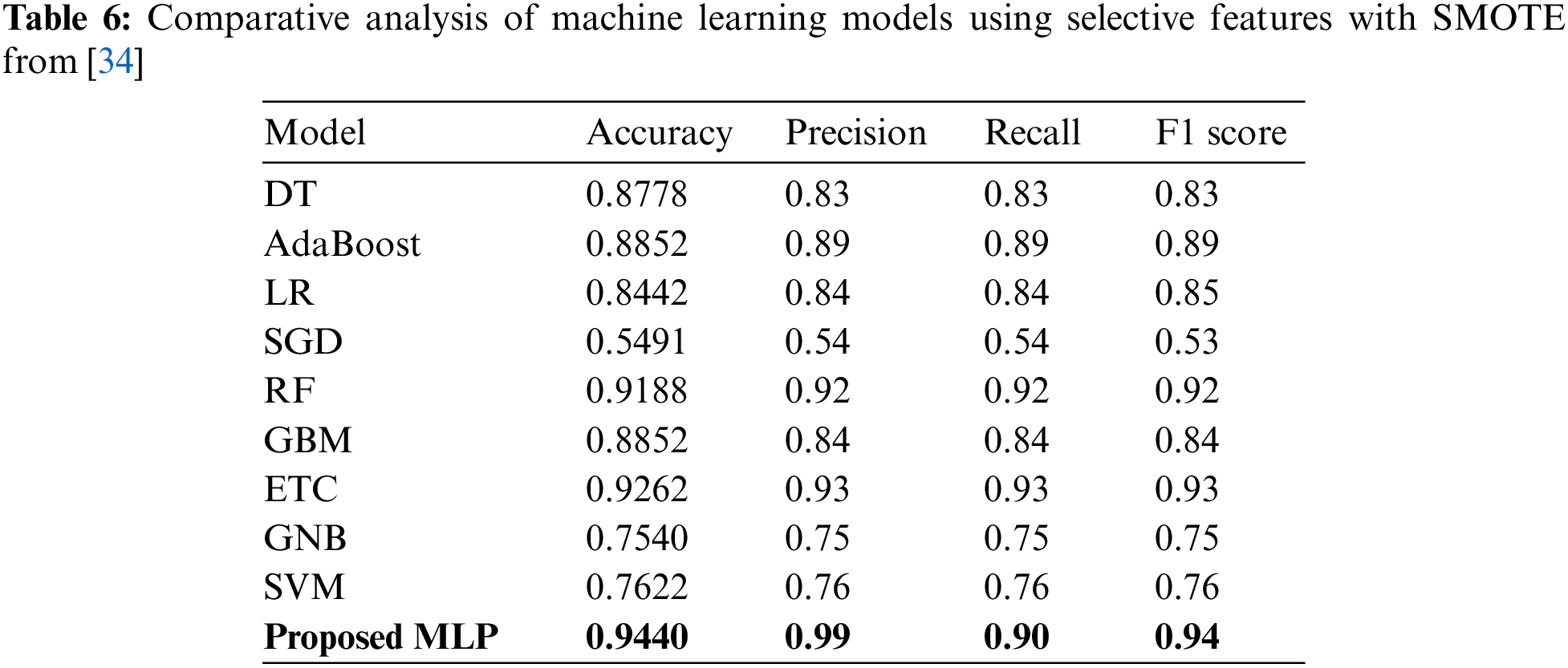
Similarly in [33], authors applied deep learning models including CNN, MLP, RNN, and LSTM for heart disease prediction. In addition, it also uses two pre-trained models including VGG16 and AlexNet for heart disease prediction. Table 7 presents findings for performance comparison. The best results are observed by CNN with 0.9289 accuracy while MLP has shown the second-highest results with a 0.9201 accuracy. Existing studies have applied various optimization techniques like suitable feature extraction techniques or feature selection techniques in combination with machine learning or deep learning classifiers applied on different datasets.
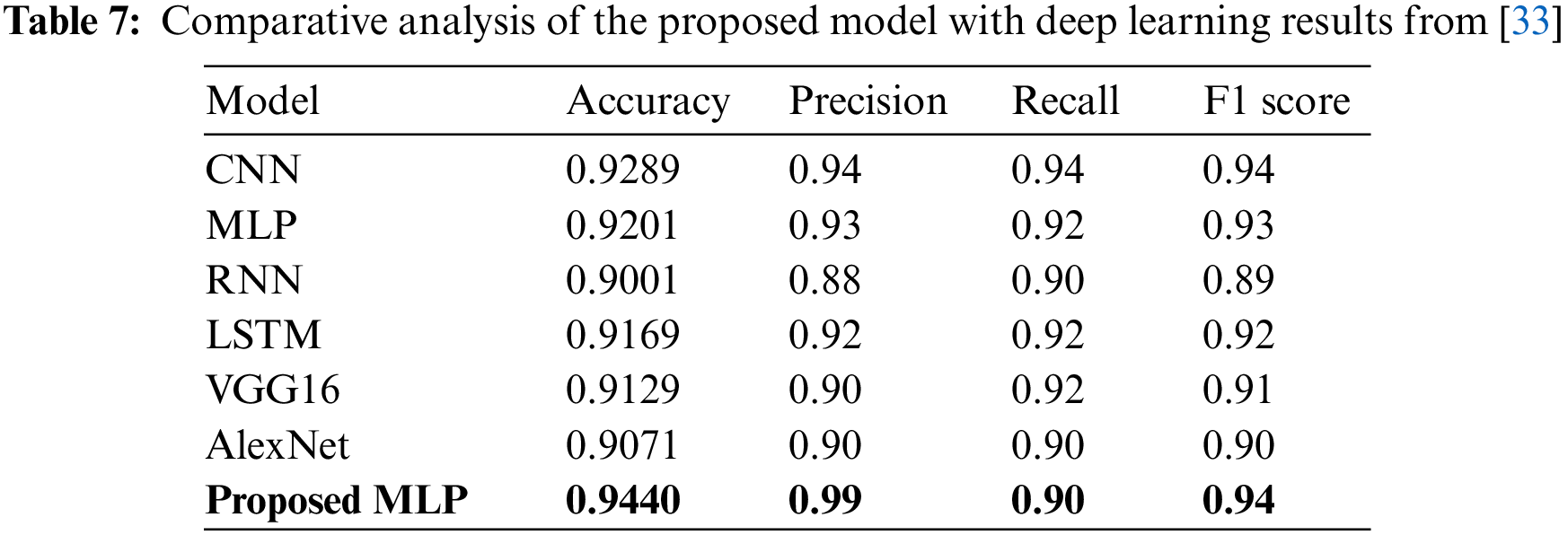
Fig. 5 shows performance comparison results of the machine and deep learning models from existing studies regarding accuracy. It is evident that the proposed MLP model outperforms existing studies and shows superior performance.
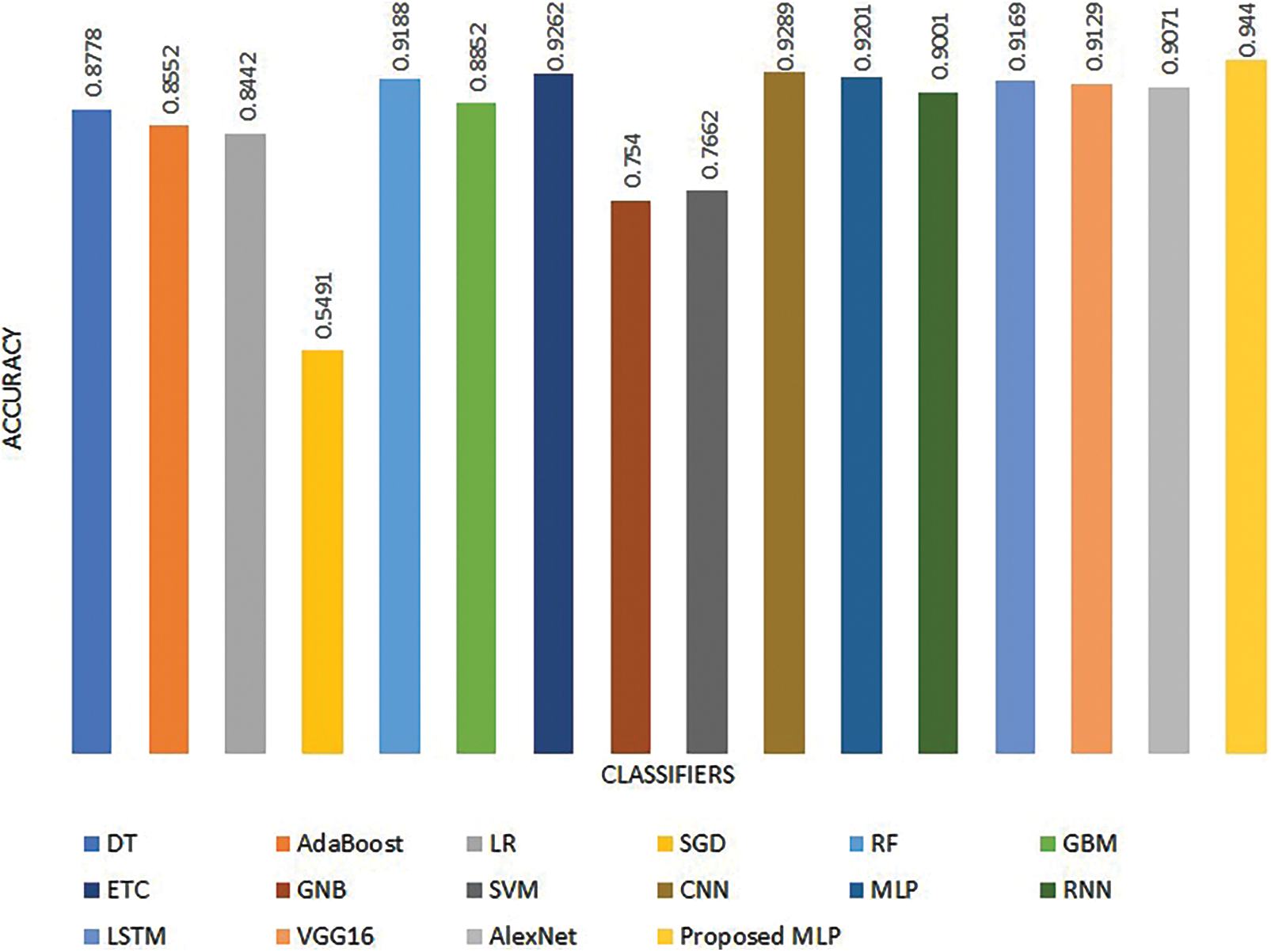
Figure 5: Performance comparison with existing studies
The discussed studies face limitations like small-sized datasets where augmentation approaches are needed such as SMOTE. Some researchers applied a hybrid machine learning model based on individual weak learners with feature selection techniques, which are not generalized enough. The current study does not utilize any data augmentation techniques and proposes an MLP architecture that shows robust and efficient results comparable to previous research. The results of the current study prove that the proposed MLP is more precise than the existing ones in terms of accuracy, precision, recall, and F1 score using the ECG dataset. To prevent heart diseases from becoming severe, early detection and prediction of these disorders are crucial. Furthermore, it makes it possible to build relationships between important facts, including patterns between medical parameters associated with heart disease.
Cardiovascular diseases have become one of the leading causes of death around the world and the number of patients has increased gradually. The ECG data is used for diagnosing heart disease by medical experts. However, the diagnosis is error-prone, subjective, and time-consuming. Although machine learning approaches have become prevalent to assist medical experts in heart disease detection, they face several challenges. For the most part, such approaches either rely on image processing or require manual feature extraction for machine learning models. Consequently, the use of ECG signals remains an under-explored research area. This study proposes a multilayer perceptron for heart disease detection using ECG signals and performs several experiments using machine learning and deep learning models. Results confirm that the proposed MLP shows superb performance with a 94.40% heart disease detection rate and outperforms CNN, RNN, LSTM, LR, ETC, SGD, RF, and GNB, which are used for experiments. MLPs work well for classification prediction issues where the inputs have been given a class or label. It also shows better accuracy than existing approaches that use feature extraction, data augmentation, and selective features. The proposed model is both efficient and robust and has lower computational complexity.
Funding Statement: This research work receives no external funding. Funding is done by author Abdullah Mohamed.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Seckeler, M. D., Hoke, T. R. (2011). The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clinical Epidemiology, 3(1), 67–84. [Google Scholar] [PubMed]
2. Gaziano, T. A., Bitton, A., Anand, S., Abrahams-Gessel, S., Murphy, A. (2010). Growing epidemic of coronary heart disease in low-and middle-income countries. Current Problems in Cardiology, 35(2), 72–115. https://doi.org/10.1016/j.cpcardiol.2009.10.002 [Google Scholar] [PubMed] [CrossRef]
3. Ahmed, H., Younis, E. M., Hendawi, A., Ali, A. A. (2020). Heart disease identification from patients’ social posts, machine learning solution on spark. Future Generation Computer Systems, 111(12), 714–722. https://doi.org/10.1016/j.future.2019.09.056 [Google Scholar] [CrossRef]
4. Alfaras, M., Soriano, M. C., Ortín, S. (2019). A fast machine learning model for ECG-based heartbeat classification and arrhythmia detection. Frontiers in Physics, 103, e215. https://doi.org/10.3389/fphy.2019.00103 [Google Scholar] [CrossRef]
5. Siontis, K. C., Noseworthy, P. A., Attia, Z. I., Friedman, P. A. (2021). Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nature Reviews Cardiology, 18(7), 465–478. https://doi.org/10.1038/s41569-020-00503-2 [Google Scholar] [PubMed] [CrossRef]
6. Venkatesan, C., Karthigaikumar, P., Paul, A., Satheeskumaran, S., Kumar, R. (2018). ECG signal preprocessing and SVM classifier-based abnormality detection in remote healthcare applications. IEEE Access, 6, 9767–9773. https://doi.org/10.1109/ACCESS.2018.2794346 [Google Scholar] [CrossRef]
7. Gawali, D. H., Wadhai, V. M. (2014). Implementation of ECG sensor for real time signal processing applications. 2014 International Conference on Advances in Electronics Computers and Communications, pp. 1–3. Reva University, Bangalore, India, IEEE. [Google Scholar]
8. Jeon, T., Kim, B., Jeon, M., Lee, B. G. (2014). Implementation of a portable device for real-time ECG signal analysis. BioMedical Engineering OnLine, 13(1), 1–13. https://doi.org/10.1186/1475-925X-13-S1-I1 [Google Scholar] [CrossRef]
9. Sadhukhan, D., Pal, S., Mitra, M. (2018). Automated identification of myocardial infarction using harmonic phase distribution pattern of ECG data. IEEE Transactions on Instrumentation and Measurement, 67(10), 2303–2313. https://doi.org/10.1109/TIM.2018.2816458 [Google Scholar] [CrossRef]
10. Oh, S. L., Hagiwara, Y., Raghavendra, U., Yuvaraj, R., Arunkumar, N. et al. (2020). A deep learning approach for Parkinson’s disease diagnosis from EEG signals. Neural Computing and Applications, 32(15), 10927–10933. https://doi.org/10.1007/s00521-018-3689-5 [Google Scholar] [CrossRef]
11. Li, H., Pan, Y., Zhao, J., Zhang, L. (2021). Skin disease diagnosis with deep learning: A review. Neurocomputing, 464(6), 364–393. https://doi.org/10.1016/j.neucom.2021.08.096 [Google Scholar] [CrossRef]
12. Lih, O. S., Jahmunah, V., San, T. R., Ciaccio, E. J., Yamakawa, T. et al. (2020). Comprehensive electrocardiographic diagnosis based on deep learning. Artificial Intelligence in Medicine, 103(4), 101789. https://doi.org/10.1016/j.artmed.2019.101789 [Google Scholar] [PubMed] [CrossRef]
13. Hasan, N. I., Bhattacharjee, A. (2019). Deep learning approach to cardiovascular disease classification employing modified ECG signal from empirical mode decomposition. Biomedical Signal Processing and Control, 52(12), 128–140. https://doi.org/10.1016/j.bspc.2019.04.005 [Google Scholar] [CrossRef]
14. Tan, L., Yu, K., Bashir, A. K., Cheng, X., Ming, F. et al. (2021). Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: A deep learning approach. Neural Computing and Applications, 1–14. [Google Scholar]
15. Madani, A., Ong, J. R., Tibrewal, A., Mofrad, M. R. (2018). Deep echocardiography: Data-efficient supervised and semi-supervised deep learning towards automated diagnosis of cardiac disease. NPJ Digital Medicine, 1(1), 59. https://doi.org/10.1038/s41746-018-0065-x [Google Scholar] [PubMed] [CrossRef]
16. Alizadehsani, R., Abdar, M., Roshanzamir, M., Khosravi, A., Kebria, P. M. et al. (2019). Machine learning-based coronary artery disease diagnosis: A comprehensive review. Computers in Biology and Medicine, 111(1), 103346. https://doi.org/10.1016/j.compbiomed.2019.103346 [Google Scholar] [PubMed] [CrossRef]
17. Petersen, S. E., Abdulkareem, M., Leiner, T. (2019). Artificial intelligence will transform cardiac imaging—opportunities and challenges. Frontiers in Cardiovascular Medicine, 6, 133. https://doi.org/10.3389/fcvm.2019.00133 [Google Scholar] [PubMed] [CrossRef]
18. Waigi, D., Choudhary, D. S., Fulzele, D. P., Mishra, D. (2020). Predicting the risk of heart disease using advanced machine learning approach. European Journal of Molecular & Clinical Medicine, 7(7), 1638–1645. [Google Scholar]
19. Mora, M. A., Saarijärvi, M., Sparud-Lundin, C., Moons, P., Bratt, E. L. (2020). Empowering young persons with congenital heart disease: Using intervention mapping to develop a transition program-the STEPSTONES project. Journal of Pediatric Nursing, 50(7), e8–e17. https://doi.org/10.1016/j.pedn.2019.09.021 [Google Scholar] [PubMed] [CrossRef]
20. Khalil, M., Adib, A. (2020). An end-to-end multi-level wavelet convolutional neural networks for heart diseases diagnosis. Neurocomputing, 417(5), 187–201. https://doi.org/10.1016/j.neucom.2020.07.056 [Google Scholar] [CrossRef]
21. Li, J. P., Haq, A. U., Din, S. U., Khan, J., Khan, A. et al. (2020). Heart disease identification method using machine learning classification in e-healthcare. IEEE Access, 8, 107562–107582. https://doi.org/10.1109/ACCESS.2020.3001149 [Google Scholar] [CrossRef]
22. Khanna, D., Sahu, R., Baths, V., Deshpande, B. (2015). Comparative study of classification techniques (SVM, logistic regression and neural networks) to predict the prevalence of heart disease. International Journal of Machine Learning and Computing, 5(5), 414–419. https://doi.org/10.7763/IJMLC.2015.V5.544 [Google Scholar] [CrossRef]
23. Acharya, A. (2017). Comparative study of machine learning algorithms for heart disease prediction. https://www.theseus.fi/handle/10024/124622 [Google Scholar]
24. Kumar, M. N., Koushik, K. V. S., Deepak, K. (2018). Prediction of heart diseases using data mining and machine learning algorithms and tools. International Journal of Scientific Research in Computer Science, Engineering and Information Technology, 3(3), 887–898. [Google Scholar]
25. Kwon, J. M., Kim, K. H., Jeon, K. H., Park, J. (2019). Deep learning for predicting in-hospital mortality among heart disease patients based on echocardiography. Echocardiography, 36(2), 213–218. https://doi.org/10.1111/echo.14220 [Google Scholar] [PubMed] [CrossRef]
26. Jonnalagadda, S. R., Adupa, A. K., Garg, R. P., Corona-Cox, J., Shah, S. J. (2017). Text mining of the electronic health record: An information extraction approach for automated identification and subphenotyping of HFpEF patients for clinical trials. Journal of Cardiovascular Translational Research, 10(3), 313–321. https://doi.org/10.1007/s12265-017-9752-2 [Google Scholar] [PubMed] [CrossRef]
27. Jonnagaddala, J., Liaw, S. T., Ray, P., Kumar, M., Chang, N. W. et al. (2015). Coronary artery disease risk assessment from unstructured electronic health records using text mining. Journal of Biomedical Informatics, 58(9063), S203–S210. https://doi.org/10.1016/j.jbi.2015.08.003 [Google Scholar] [PubMed] [CrossRef]
28. Tougui, I., Jilbab, A., El Mhamdi, J. (2020). Heart disease classification using data mining tools and machine learning techniques. Health and Technology, 10(5), 1137–1144. https://doi.org/10.1007/s12553-020-00438-1 [Google Scholar] [CrossRef]
29. Davoodi, R., Moradi, M. H. (2018). Mortality prediction in intensive care units (ICUs) using a deep rule-based fuzzy classifier. Journal of Biomedical Informatics, 79(11), 48–59. https://doi.org/10.1016/j.jbi.2018.02.008 [Google Scholar] [PubMed] [CrossRef]
30. Fazlic, L. B., Hallawa, A., Schmeink, A., Peine, A., Martin, L. et al. (2019). A novel NLP-fuzzy system prototype for information extraction from medical guidelines. 2019 42nd International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), pp. 1025–1030. Opatija, Croatia, IEEE. [Google Scholar]
31. Song, C. W., Jung, H., Chung, K. (2019). Development of a medical big-data mining process using topic modeling. Cluster Computing, 22(S1), 1949–1958. https://doi.org/10.1007/s10586-017-0942-0 [Google Scholar] [CrossRef]
32. Mohan, S., Thirumalai, C., Srivastava, G. (2019). Effective heart disease prediction using hybrid machine learning techniques. IEEE Access, 7, 81542–81554. https://doi.org/10.1109/ACCESS.2019.2923707 [Google Scholar] [CrossRef]
33. Umer, M., Sadiq, S., Karamti, H., Karamti, W., Majeed, R. et al. (2022). IoT based smart monitoring of patients’ with acute heart failure. Sensors, 22(7), 2431. https://doi.org/10.3390/s22072431 [Google Scholar] [PubMed] [CrossRef]
34. Ishaq, A., Sadiq, S., Umer, M., Ullah, S., Mirjalili, S. et al. (2021). Improving the prediction of heart failure patients’ survival using SMOTE and effective data mining techniques. IEEE Access, 9, 39707–39716. https://doi.org/10.1109/ACCESS.2021.3064084 [Google Scholar] [CrossRef]
35. Riyaz, L., Butt, M. A., Zaman, M., Ayob, O. (2022). Heart disease prediction using machine learning techniques: A quantitative review. International Conference on Innovative Computing and Communications: Proceedings of ICICC 2021, vol. 3, pp. 81–94. Singapore, Springer. [Google Scholar]
36. Muzammal, M., Talat, R., Sodhro, A. H., Pirbhulal, S. (2020). A multi-sensor data fusion enabled ensemble approach for medical data from body sensor networks. Information Fusion, 53, 155–164. https://doi.org/10.1016/j.inffus.2019.06.021 [Google Scholar] [CrossRef]
37. Nguyen, T., Khosravi, A., Creighton, D., Nahavandi, S. (2015). Classification of healthcare data using genetic fuzzy logic system and wavelets. Expert Systems with Applications, 42(4), 2184–2197. https://doi.org/10.1016/j.eswa.2014.10.027 [Google Scholar] [CrossRef]
38. Zhang, J., Lafta, R. L., Tao, X., Li, Y., Chen, F. et al. (2017). Coupling a fast fourier transformation with a machine learning ensemble model to support recommendations for heart disease patients in a telehealth environment. IEEE Access, 5, 10674–10685. https://doi.org/10.1109/ACCESS.2017.2706318 [Google Scholar] [CrossRef]
39. Long, N. C., Meesad, P., Unger, H. (2015). A highly accurate firefly based algorithm for heart disease prediction. Expert Systems with Applications, 42(21), 8221–8231. https://doi.org/10.1016/j.eswa.2015.06.024 [Google Scholar] [CrossRef]
40. Darmawahyuni, A., Nurmaini, S., Caesarendra, W., Bhayyu, V., Rachmatullah, M. N. (2019). Deep learning with a recurrent network structure in the sequence modeling of imbalanced data for ECG-rhythm classifier. Algorithms, 12(6), 118. https://doi.org/10.3390/a12060118 [Google Scholar] [CrossRef]
41. Cheng, C. H., Wong, K. L., Chin, J. W., Chan, T. T., So, R. H. (2021). Deep learning methods for remote heart rate measurement: A review and future research agenda. Sensors, 21(18), 6296. https://doi.org/10.3390/s21186296 [Google Scholar] [PubMed] [CrossRef]
42. Massaro, A., Maritati, V., Savino, N., Galiano, A. (2018). Neural networks for automated smart health platforms oriented on heart predictive diagnostic big data systems. 2018 AEIT International Annual Conference, pp. 1–5. Piazzale Aldo, Moro, Rome, Italy, IEEE. [Google Scholar]
43. Massaro, A., Ricci, G., Selicato, S., Raminelli, S., Galiano, A. (2020). Decisional support system with artificial intelligence oriented on health prediction using a wearable device and big data. 2020 IEEE International Workshop on Metrology for Industry 4.0 & IoT, pp. 718–723. The Netherlands, IEEE. [Google Scholar]
44. Liu, P., Sun, X., Han, Y., He, Z., Zhang, W. et al. (2022). Arrhythmia classification of LSTM autoencoder based on time series anomaly detection. Biomedical Signal Processing and Control, 71(4), 103228. https://doi.org/10.1016/j.bspc.2021.103228 [Google Scholar] [CrossRef]
45. de Falco, I., de Pietro, G., Della Cioppa, A., Sannino, G., Scafuri, U. et al. (2019). Evolution-based configuration optimization of a deep neural network for the classification of obstructive sleep apnea episodes. Future Generation Computer Systems, 98(7553), 377–391. https://doi.org/10.1016/j.future.2019.01.049 [Google Scholar] [CrossRef]
46. Sannino, G., de Pietro, G. (2018). A deep learning approach for ECG-based heartbeat classification for arrhythmia detection. Future Generation Computer Systems, 86(1), 446–455. https://doi.org/10.1016/j.future.2018.03.057 [Google Scholar] [CrossRef]
47. Haleem, M. S., Castaldo, R., Pagliara, S. M., Petretta, M., Salvatore, M. et al. (2021). Time adaptive ECG driven cardiovascular disease detector. Biomedical Signal Processing and Control, 70(12), 102968. https://doi.org/10.1016/j.bspc.2021.102968 [Google Scholar] [CrossRef]
48. Tripathy, D. (2021). ECG dataset. https://www.kaggle.com/datasets/devavratatripathy/ecg-dataset [Google Scholar]
49. Shahzad, H. F., Rustam, F., Flores, E. S., Luís Vidal Mazón, J., de la Torre Diez, I. et al. (2022). A review of image processing techniques for deepfakes. Sensors, 22(12), 4556. [Google Scholar] [PubMed]
50. Ashraf, I., Hur, S., Park, Y. (2019). Application of deep convolutional neural networks and smartphone sensors for indoor localization. Applied Sciences, 9(11), 2337. https://doi.org/10.3390/app9112337 [Google Scholar] [CrossRef]
51. Mujahid, M., Lee, E., Rustam, F., Washington, P. B., Ullah, S. et al. (2021). Sentiment analysis and topic modeling on tweets about online education during COVID-19. Applied Sciences, 11(18), 8438. https://doi.org/10.3390/app11188438 [Google Scholar] [CrossRef]
52. Reshi, A. A., Ashraf, I., Rustam, F., Shahzad, H. F., Mehmood, A. et al. (2021). Diagnosis of vertebral column pathologies using concatenated resampling with machine learning algorithms. PeerJ Computer Science, 7(6), e547. https://doi.org/10.7717/peerj-cs.547 [Google Scholar] [PubMed] [CrossRef]
53. Umer, M., Ashraf, I., Ullah, S., Mehmood, A., Choi, G. S. (2022). COVINet: A convolutional neural network approach for predicting COVID-19 from chest X-ray images. Journal of Ambient Intelligence and Humanized Computing, 13(1), 1–13. https://doi.org/10.1007/s12652-021-02917-3 [Google Scholar] [PubMed] [CrossRef]
54. Bornschein, J., Visin, F., Osindero, S. (2020). Small data, big decisions: Model selection in the small-data regime. International Conference on Machine Learning, pp. 1035–1044. [Google Scholar]
55. Skourt, B. A., El Hassani, A., Majda, A. (2022). Mixed-pooling-dropout for convolutional neural network regularization. Journal of King Saud University-Computer and Information Sciences, 34(8), 4756–4762. https://doi.org/10.1016/j.jksuci.2021.05.001 [Google Scholar] [CrossRef]
56. Zhang, Y. D., Jiang, X., Wang, S. H. (2022). Fingerspelling recognition by 12-layer CNN with stochastic pooling. Mobile Networks and Applications, 14(1), 1–13. https://doi.org/10.1007/s11036-021-01900-8 [Google Scholar] [CrossRef]
57. Sunija, A. P., Kar, S., Gayathri, S., Gopi, V. P., Palanisamy, P. (2021). OctNET: A lightweight CNN for retinal disease classification from optical coherence tomography images. Computer Methods and Programs in Biomedicine, 200(8), 105877. https://doi.org/10.1016/j.cmpb.2020.105877 [Google Scholar] [PubMed] [CrossRef]
58. Che, H., Brown, L. G., Foran, D. J., Nosher, J. L., Hacihaliloglu, I. (2021). Liver disease classification from ultrasound using multi-scale CNN. International Journal of Computer Assisted Radiology and Surgery, 16(9), 1537–1548. https://doi.org/10.1007/s11548-021-02414-0 [Google Scholar] [PubMed] [CrossRef]
59. Umer, M., Sadiq, S., Ahmad, M., Ullah, S., Choi, G. S. et al. (2020). A novel stacked CNN for malarial parasite detection in thin blood smear images. IEEE Access, 8, 93782–93792. https://doi.org/10.1109/ACCESS.2020.2994810 [Google Scholar] [CrossRef]
60. Deepak, S., Ameer, P. M. (2019). Brain tumor classification using deep CNN features via transfer learning. Computers in Biology and Medicine, 111(3), 103345. https://doi.org/10.1016/j.compbiomed.2019.103345 [Google Scholar] [PubMed] [CrossRef]
61. Peng, H., Li, J., He, Y., Liu, Y., Bao, M. (2018). Large-scale hierarchical text classification with recursively regularized deep graph-CNN. Proceedings of the 2018 World Wide Web Conference, pp. 1063–1072. Lyon, France. [Google Scholar]
62. Banerjee, I., Ling, Y., Chen, M. C., Hasan, S. A., Langlotz, C. P. et al. (2019). Comparative effectiveness of convolutional neural network (CNN) and recurrent neural network (RNN) architectures for radiology text report classification. Artificial Intelligence in Medicine, 97(2), 79–88. https://doi.org/10.1016/j.artmed.2018.11.004 [Google Scholar] [PubMed] [CrossRef]
63. Zhang, Y., Zhang, X., Zhu, W. (2021). ANC: Attention network for COVID-19 explainable diagnosis based on convolutional block attention module. Computer Modeling in Engineering & Sciences, 127(3), 1037–1058. https://doi.org/10.32604/cmes.2021.015807 [Google Scholar] [CrossRef]
64. Baloglu, U. B., Talo, M., Yildirim, O., San Tan, R., Acharya, U. R. (2019). Classification of myocardial infarction with multi-lead ECG signals and deep CNN. Pattern Recognition Letters, 122(3), 23–30. https://doi.org/10.1016/j.patrec.2019.02.016 [Google Scholar] [CrossRef]
65. Xiong, Z., Stiles, M. K., Zhao, J. (2017). Robust ECG signal classification for detection of atrial fibrillation using a novel neural network. 2017 Computing in Cardiology (CinC), pp. 1–4. Rennes, France, IEEE. [Google Scholar]
66. Ullah, A., Rehman, S. U., Tu, S., Mehmood, R. M., Ehatisham-Ul-Haq, M. (2021). A hybrid deep CNN model for abnormal arrhythmia detection based on cardiac ECG signal. Sensors, 21(3), 951. https://doi.org/10.3390/s21030951 [Google Scholar] [PubMed] [CrossRef]
67. Lin, J. C. W., Shao, Y., Djenouri, Y., Yun, U. (2021). ASRNN: A recurrent neural network with an attention model for sequence labeling. Knowledge-Based Systems, 212(1), 106548. https://doi.org/10.1016/j.knosys.2020.106548 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools