
 | Computer Modeling in Engineering & Sciences |  |
DOI: 10.32604/cmes.2021.017740
ARTICLE
Fluid and Osmotic Pressure Balance and Volume Stabilization in Cells
Dedicated to Professor Karl Stark Pister for his 95th birthday
Department of Mechanical Engineering, Stanford University, Stanford, 94305, CA, USA
*Corresponding Author: Peter M. Pinsky. Email: pinsky@stanford.edu
Received: 02 June 2021; Accepted: 26 July 2021
Abstract: A fundamental problem for cells with their fragile membranes is the control of their volume. The primordial solution to this problem is the active transport of ions across the cell membrane to modulate the intracellular osmotic pressure. In this work, a theoretical model of the cellular pump-leak mechanism is proposed within the general framework of linear nonequilibrium thermodynamics. The model is expressed with phenomenological equations that describe passive and active ionic transport across cell membranes, supplemented by an equation for the membrane potential that accounts for the electrogenicity of the ionic pumps. For active ionic transport, the model predicts that the intracellular fluid pressure will be balanced by the osmotic pressure and a new pressure component that arises from the active ionic fluxes. A model for the pump-leak mechanism in an idealized human cell is introduced to demonstrate the applicability of the proposed theory.
Keywords: Pump-leak mechanism; cell volume regulation; active ion transport; ion pump; membrane transport; cell mechanics; modified Kedem-Katchalsky equations; nonequilibrium thermodynamics; phenomenological equations
A cell must concentrate and protect within its interior substances that are essential for its function—DNA, proteins, amino acids and sugars. These sequestered substances, which are entrapped within the cell, introduce a special challenge for the cell. They carry significant concentrations of charge that establishes a high osmotic pressure within the cell. The osmotic pressure difference between the intracellular and extracellular media produces a tendency to swelling by water inflow across the cell membrane. Such swelling can be arrested by two processes: actively reducing the cellular ionic content through ion pumps, and by the generation of internal fluid pressure that can act to stop the flow of water. In fact, both processes will act simultaneously. However, animal cells have fragile membranes and it has generally been accepted that the primary action of the pump-leak mechanism (PLM) is the active reduction of cellular ionic content and the concomitant reduction in osmotic pressure. As a result, models that have been developed to explain the PLM have concentrated on osmotic stabilization due to ion pumping [1–5]. The role of cellular fluid pressure, generated by the resistance to expansion of the cell membrane–cortex has received less attention [6–8]. A general framework for modeling the PLM including the role of fluid pressure is proposed in this work.
The movement of ions across the cell membrane occurs passively and actively. Passive transport occurs by diffusion and convection and requires no energy input. Active transport processes move ions against concentration gradients through the expenditure of energy. Both processes are important and the interplay between them, controlled by feedback [9], facilitates the regulation of cell volume, preventing potential cell rupture or collapse under changing conditions.
The cytoplasm (i.e., intracellular fluid) contains charged macromolecules and metabolites that are confined to the cell interior by entrapment and are impermeant with respect to the cell membrane—all such impermeant charged macromolecules will be referred to as fixed charges for brevity. The fixed charges localize mobile ions which form an electrical double layer of counterions and coions that screens the electric field. The resulting ion concentrations establish the osmotic pressure within the cell. If the extracellular solution has, for example, lower ionic concentrations, water will flow across the cell membrane and into the cell by osmosis, causing the cell to swell. It is the fixed charges that are essentially responsible for the swelling tendency of the cell. Active ion transporters (e.g., the Na+ ion pump) that convert energy from various sources, including adenosine triphosphate (ATP), are located in the cell membrane and produce outward and inward fluxes of ions that modulate the osmotic and fluid pressures to arrest and reverse cellular swelling [10].
The linear theory of nonequilibrium thermodynamics has been widely employed to model passive transport processes. The approach asserts the existence of a dissipation function which describes the rate of change of entropy production. It is expressed as the sum of a set of flux and conjugate (driving) force products. For example, the classical study of Kedem et al. [11,12] used this approach to obtain the flux definitions that are conjugate to the fluid and osmotic pressures for a non-electrolyte solution. Then, considering near-equilibrium, a linear relationship between each flux and all conjugate forces is postulated. The result is a set of phenomenological equations that describes all interactions between the solvent and solutes and which is expressed with transport coefficients that have the significant merit of being amenable to experimental measurement.
When the solutes crossing the membrane are charged, the nonequilibrium thermodynamic description is more challenging and the system exhibits new features. A very general framework based on linear nonequilibrium thermodynamics has been given by Kedem et al. [13] and includes many electrokinetic phenomena within its scope. More recently, Li [14] proposed phenomenological equations for the passive transport of ionic solutions that account for electrostatic interactions between ions. This was extended by Cheng et al. [15] to account for fixed charges associated with proteoglycans for application to the corneal endothelium. The latter work identified a fluid pressure component that appears during active ion pumping and which must be considered in the balance of fluid and osmotic pressure. The goal of the present paper is to describe the temporal and steady state behavior of the pump-leak system utilizing the fully general framework of nonequilibrium thermodynamics.
We start with a brief review of the development of phenomenological equations for passive transport across a semipermeable membrane separating two ionic solutions, one of which contains impermeant charged macromolecules. Extension of the theory for active ion transport is then described, including derivation of the generalized pressure conjugate to the active ion flux. Analytical steady state solutions are obtained for both the passive and active cases and considering an intracellular binary electrolyte solution. Solutions to the temporal problem are obtained numerically.
To illustrate the scope and features of the theory, a numerical study of a highly idealized model of a human cell is introduced. The cell is in suspension (without attachments) and is subjected to a sequence of hypotonic and hypertonic shocks. For simplicity, the intracellular and extracellular solutions are taken to be binary electrolyte solutions and the phenomenological equations are suitably specialized. The model is first applied to analyzing the response of the cell model with only passive transport. In a second analysis, active cation transport is initiated when a signal based on membrane tension is received. This simulation provides the time course of the cell radius, fluid pressure, osmotic pressures, and other quantities. The numerical results suggest that the model replicates the essential features of the PLM and that the fluid pressure component arising from the active ion flux is an essential factor in the balance of fluid and osmotic pressures, including under steady state conditions.
2 Modified Kedem-Katchalsky Equations for Passive Transport
The Kedem and Katchalsky (KK) phenomenological equations [11–12,16,17] are based on nonequilibrium thermodynamics and describe the transport of water and solutes across a semipermeable membrane separating two non-electrolyte solutions. They take the form
and
where Jv is the volume flow and Jk is the solute molar flux. In (1), ΔP and Δ Ck are the fluid pressure and solute concentration differences across the membrane, respectively, Lp is the hydraulic conductivity, σ k is the reflection coefficient for species k, R is the gas constant and T is the temperature. In (2),
For electrolyte solutions, the transport equations should account for the electrostatic effects of the fixed and mobile ion charges. The procedure to obtain suitable modified KK phenomenological equations [14,15] is briefly reviewed as follows. The chemical potential of water with mole fraction Xw is μ w = ν wP + RTlnXw, where
where Nspecies is the number of ion species. For brevity, sums over all ionic species will henceforward be indicated as
where ν k is the partial volume of the ion, zk is the valence number, F is the Faraday constant and ψ is the electrostatic potential.
With reference to a biological cell, ion concentrations in the intracellular fluid are denoted Ck and in the extracellular fluid
where ΔP = Pin − Pout is the membrane fluid pressure difference. Similarly,
where Δ ψ = ψ in − ψ out is the membrane potential difference. We will use the linearized form1 of (6)
where is the average ionic concentration through the membrane.
The dissipation function Φ, which measures the rate of entropy production for irreversible processes, is expressed as [11,12]
where Jw and Jk are the water and ion molar fluxes, respectively, and where
and
The ion exchange flux JDk may be interpreted as the velocity of ion k relative to the solvent. By direct manipulation of (8), it may be shown [15] that
where the conjugate forces Xv and Xs are given by
and
By assuming a dilute solution such that νk<< 1 and
and
The flows defined in (9) and (10) are now expressed as phenomenological equations having the form
where Lp is the hydraulic conductivity and σ k is the reflection coefficient of species k. LDsk are permeability coefficients which satisfy the Onsager reciprocal relation such that LDsk = LDks, reducing the number of independent coefficients.
Under the assumption of a dilute solution we have
where the solute permeability coefficient
with
and likewise employing (15) in (19) gives the ion molar flux
Eqs. (20) and (21) are modified forms of the KK Eqs. (1) and (2) that extends their application to electrolyte solutions by accounting for the membrane potential
An additional condition is needed to determine the membrane potential
and
The electric current I due to passive transport of ions through channels is given by [2,5]
where Cmem is the membrane capacitance. It is assumed that
Employing (21) in (25) and solving for Δ ψ gives
Finally, imposing the electroneutrality conditions (22) and (23), leads to
Note that this expression for F
This steady state result can also be found directly from (21) when
For subsequent use, the above theory is next specialized to the case of a NaCl binary electrolyte. The cation, anion and fixed charge concentrations are denoted C1, C2 and Cf, respectively, and have valences
and the ion fluxes (21) become
where the osmotic pressure Δ Π is
and membrane potential Δ ψ is, from (27),
Before proceeding to the case of active ion transport, we establish that the model for passive transport recovers the Donnan equilibrium pressure and concentrations. It is first established that, at equilibrium, the model predicts that ΔP = Δ Π and that this holds independently of all transport coefficients. With Jv = Jk = 0, it follows from (21) that
Using this result in (20) with Jv = 0 gives
This results confirms that
In order to obtain the equilibrium osmotic pressure, the binary electrolyte detailed at the end of Section 2 is employed for simplicity. At equilibrium Jv = J1 = J2 = 0 and it follows from (30) and (31) that
and the well-known Donnan osmotic pressure
The above results simply confirm that the passive transport model given by (20), (21) and (27) obtains the correct solution at equilibrium. We next consider the nonequilibrium problem of active ion transport.
4 Modified Kedem-Katchalsky Equations for Active Ionic Transport
Active ion transport operates to support cellular homeostasis and to prevent rupture of the cell membrane. Here we disregard the molecular-level description of ionic transport and introduce phenomenological equations for active ion transport obtained by treating the active ionic flux as an independent function of the cellular environment. It is reasonable to assume that the active ion fluxes are additive to the passive ionic flux [2,5], with the net flux expressed by
where
As in the passive transport case, we assume that Δ ψ is changing slowly enough that the capacitive current is negligible [2], resulting in
Replacing
At steady state, Jv = 0 and the membrane potential is
By comparing (41) to its value in the passive transport case (27) (or (42) to (28)), we can write
which defines how the membrane potential changes due to active ion transport.
At steady (nonequilibrium) state, the volume flux Jv and all ion net fluxes Jk will vanish, resulting in no net transport of ions [4]. Then passive ion transport will precisely balance active ion transport for each individual species and it follows from (38) that
But from (20) with Jv = 0 we observe that
Combining the above two equations to eliminate FΔ ψ leads to the important result that the fluid pressure ΔP is given by
where the osmotic pressure is
and where a new, additional, component of the fluid pressure pressure appears
From (46) it is seen that, at steady state, the fluid pressure
Steady state concentrations for a binary electrolyte (as described at the end of Section 2) are found as follows. Setting Jv = Jk = 0 in (38) implies
where β k (in mM units) is given by
Eliminating FΔ ψ from the two equations in (49) implies the Donnan-like relationship
An analogous condition has been reported in [18]. Solving this equation simultaneously with the electroneutrality condition
where
The fluid pressure contribution ΔPa given by (48) is independent of C1 and C2 and may be expressed as
Observe that when both active ion fluxes are zero, the osmotic pressure given by (54) reduces to the Donnan equilibrium pressure (37) and ΔPa given by (55) vanishes. These results clarify the influence of active ion fluxes at steady state on both the osmotic and fluid pressures and, as will be shown in the next section, are crucial to the osmoregulation of cell volumes.
5 A Minimal Model for Volume Osmoregulation of a Suspended Biological Cell
An application of the proposed modeling framework to cell volume osmoregulation is considered in this section, emphasizing steady state solutions for passive and active ion transport. The transient solution for osmotic shock loading is developed in Section 6. We consider a suspended biological cell that has spherical geometry. The cell cytoplasm and extracellular fluid are taken to be the binary electrolyte described at the end of Section 2. The cell will undergo volume expansion and contraction according to osmotic conditions.
Assuming that the number of lipid molecules in the membrane is conserved, it may be shown that the stretching free energy dominates the curvature free energy and the structural behavior of the cell cortex—lipid membrane system can therefore be modeled to first order as an elastic shell with area elasticity. The spherical elastic shell has a tension-free reference area
where K is the area elasticity constant. Using elementary statics, equilibrium requires
Using (57)1 in the expression for the volume flux Jv given by (29) and noting that
The cell radius
We next establish a relation between the ion concentrations and the cell radius. The total number of moles of ion species k occupying the cell is
which describes an evolution equation for the concentration Ck. The molar flux Jk has the additive form given by (38). Specializing the passive component
The membrane potential is given by (41) which, for the binary electrolyte and again using (59) to replace Jv, reduces to
The cell model framework is given by the three coupled ODEs (59) and (60), with the volume flux Jv and ion fluxes Jk given by (58) and (61), respectively, and the membrane potential by (62). This system can be solved numerically for
Starting with passive transport (
Fig. 1 depicts the equilibrium radius r computed for parameter values that are representative for human cells (see Table 1) and for variations in the fixed charge concentration Cf and extracellular ion concentration C0. Fig. 1 shows that both have an important influence on the cell equilibrium radius. The equilibrium radius r is independent of all membrane transport properties and provides a reference value for examining the effect of active ion transport, which is considered next.
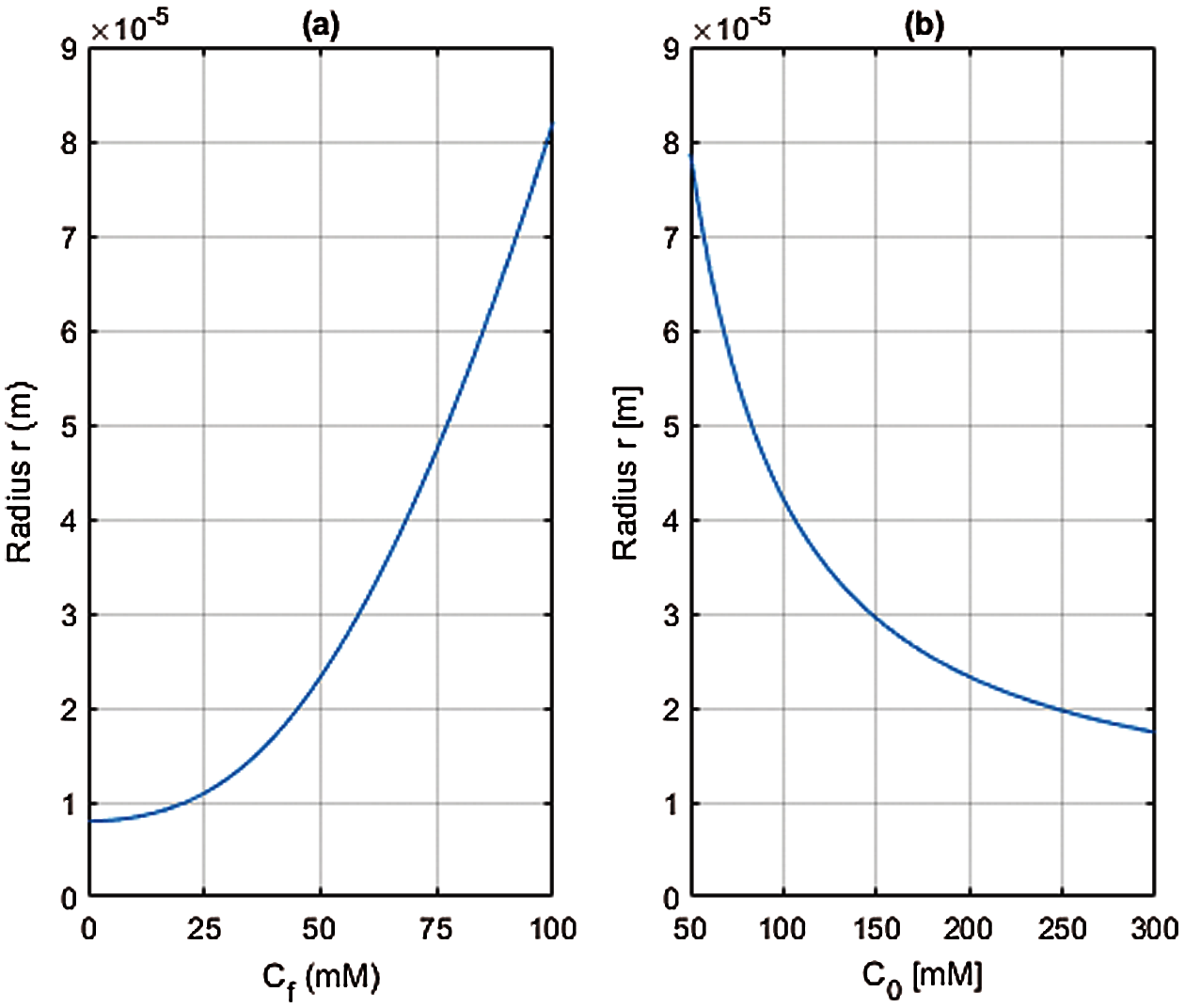
Figure 1: Steady state cell radius r computed from Eq. (63) with r0 = 8 × 10−6 m and
For active ion transport at steady (nonequilibrium) state
where Δ Π and ΔPa are evaluated using (54) and (55), respectively.
Fig. 2 examines the steady state cell radius based on (64) resulting from active transport of the cation only with
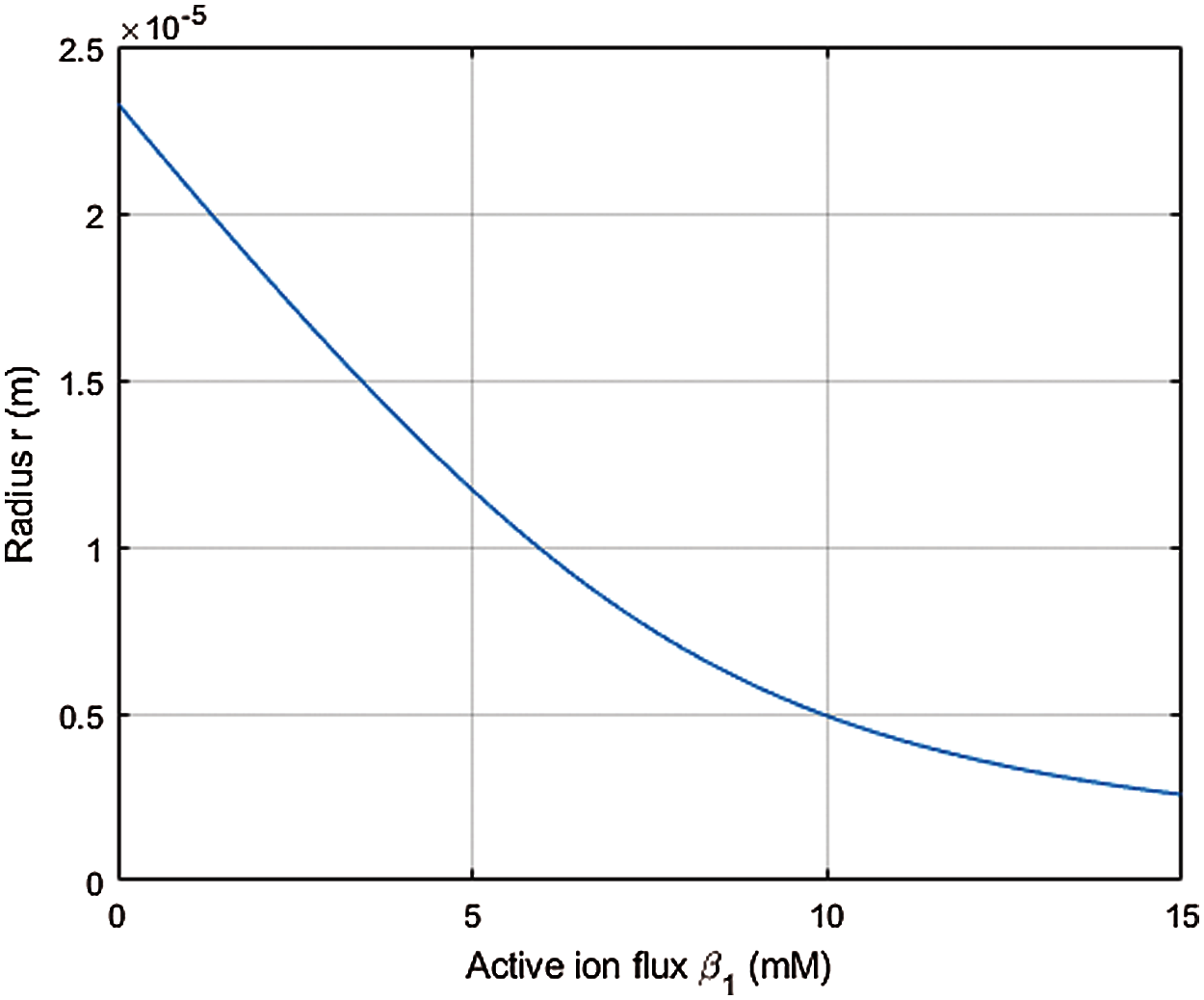
Figure 2: Steady state cell radius r (m) with active cation transport as measured by β1
The steady state values of Δ Π and
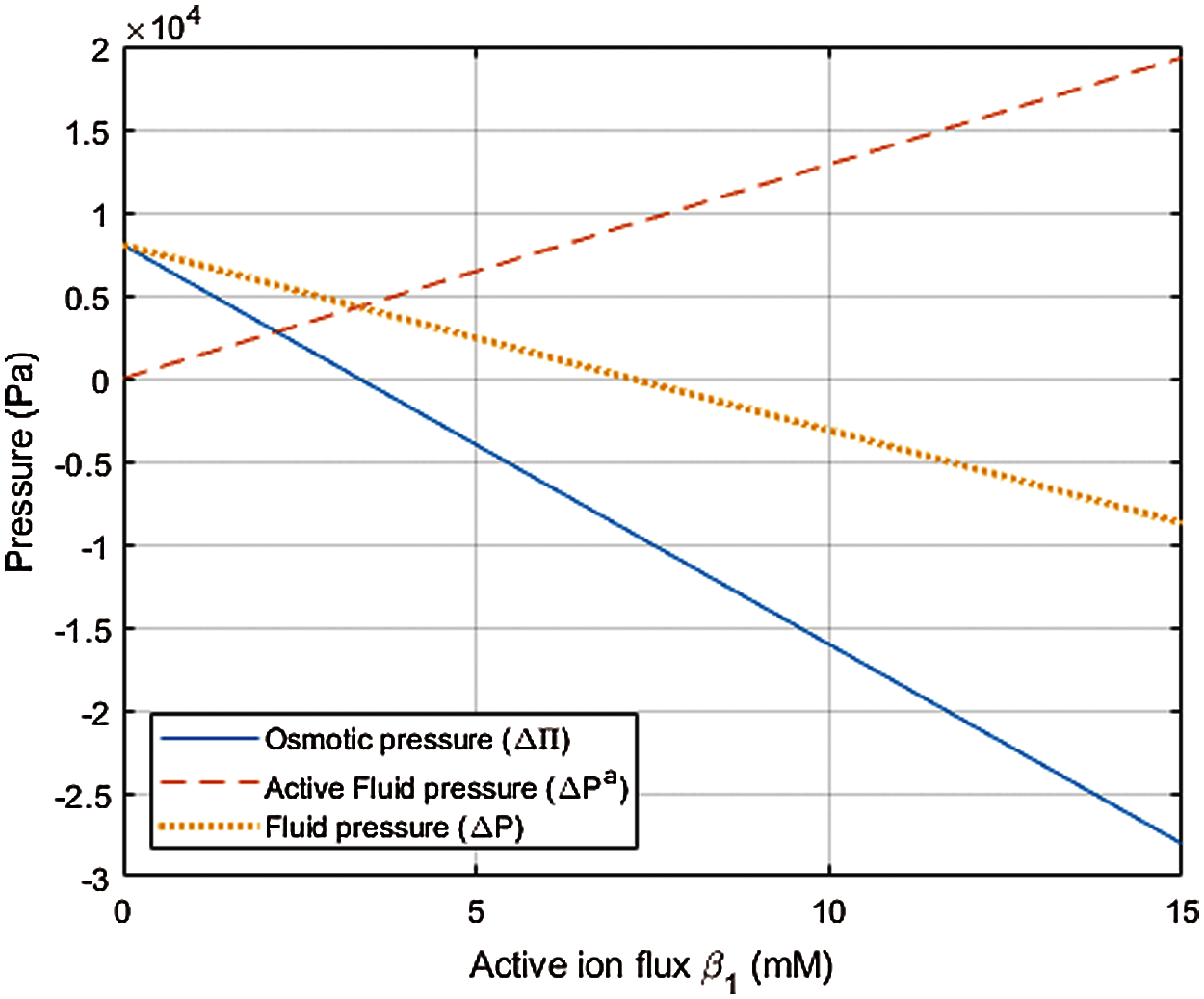
Figure 3: Plot of osmotic pressure
6 Temporal Response of the Pump-Leak Mechanism
Characterizing the details of ion channels and pumps is an active area of research that is beyond the scope of the present work. Nevertheless, a primitive example of an ion channel control model for homeostasis is provided in Section 6.3 to illustrate the ability of the proposed phenomenological equations to describe the pump-leak mechanism (PLM).
Two sets of numerical simulations are presented. In the first, the temporal response of the cell with only passive ion transport to a sequence of osmotic shocks is described in Section 6.2. Steady state results inferred from the temporal analysis are compared to the theoretical predictions given in Section 5 and provide a consistency check for the numerical implementation. In the second set of calculations, the PLM is simulated with active cation pumping. The initiation of active cation (Na+) pumping occurs when a tension-based cell membrane signal is received and subsequent changes in cell volume, fluid and osmotic pressure are reported in Section 6.3.
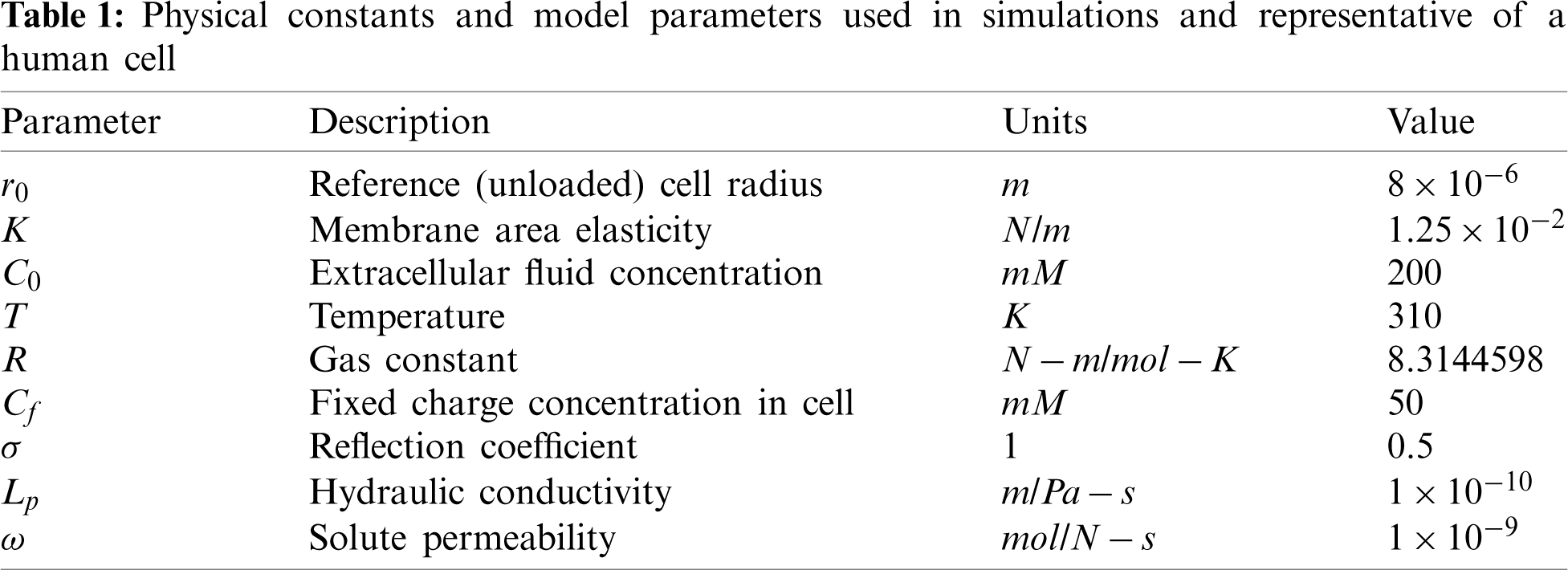
The physical constants and model parameter values given in Table 1 are used in all simulations, unless otherwise noted.
6.2 Passive Ionic Transport with Osmotic Shock
The cell was given arbitrary initial values of
where H is the Heaviside step function2. Solution of the three coupled ODEs (59) and (60), with β1 = β2 = 0 (no active ion pumping), was obtained using commercial software COMSOL Multiphysics 5.5. As seen in Fig. 4, the time interval between the initial (arbitrary) state and application of the two shocks was sufficient to allow the solution to closely approach three equilibrium states. As a validation of the temporal solution, the steady state radius and (Donnan) ionic concentrations and osmotic pressure was computed using (63), (36) and (37), respectively, at the three step levels of C0 in (65) and are given in Table 2. It may be confirmed from Fig. 4 that these values are the steady state asymptotes achieved in the temporal solution.
It may be observed in Fig. 4 that the cell radius achieves equilibrium at a slower rate than the ionic concentrations. This is expected because the rate of water transport across the membrane is controlled by the hydraulic conductivity Lp. When the hypotonic shock is applied at T = 1000 s, the volume flux Jv shown in Fig. 4d jumps to a negative value, indicating water inflow, but the slow tail of the passive inflow accounts for the slow response of the cell volume. Similarly, when the hypertonic shock is applied, the volume flux jumps to a positive value, indicating water outflow, with a time course dictated by the constant hydraulic conductivity. It may be noted from Fig. 4c that the osmotic pressure in the cell increases after the hypotonic shock and reduces after the hypertonic shock, as expected. Fig. 4b indicates that the cation and anion concentrations satisfy electroneutrality C1 − C2 = Cf = 50 mM at all times. It is remarked that the cell generates fluid pressure (not shown) by virtue of the elastic cortex—lipid membrane system and at steady state it is indeed in Donnan equilibrium.
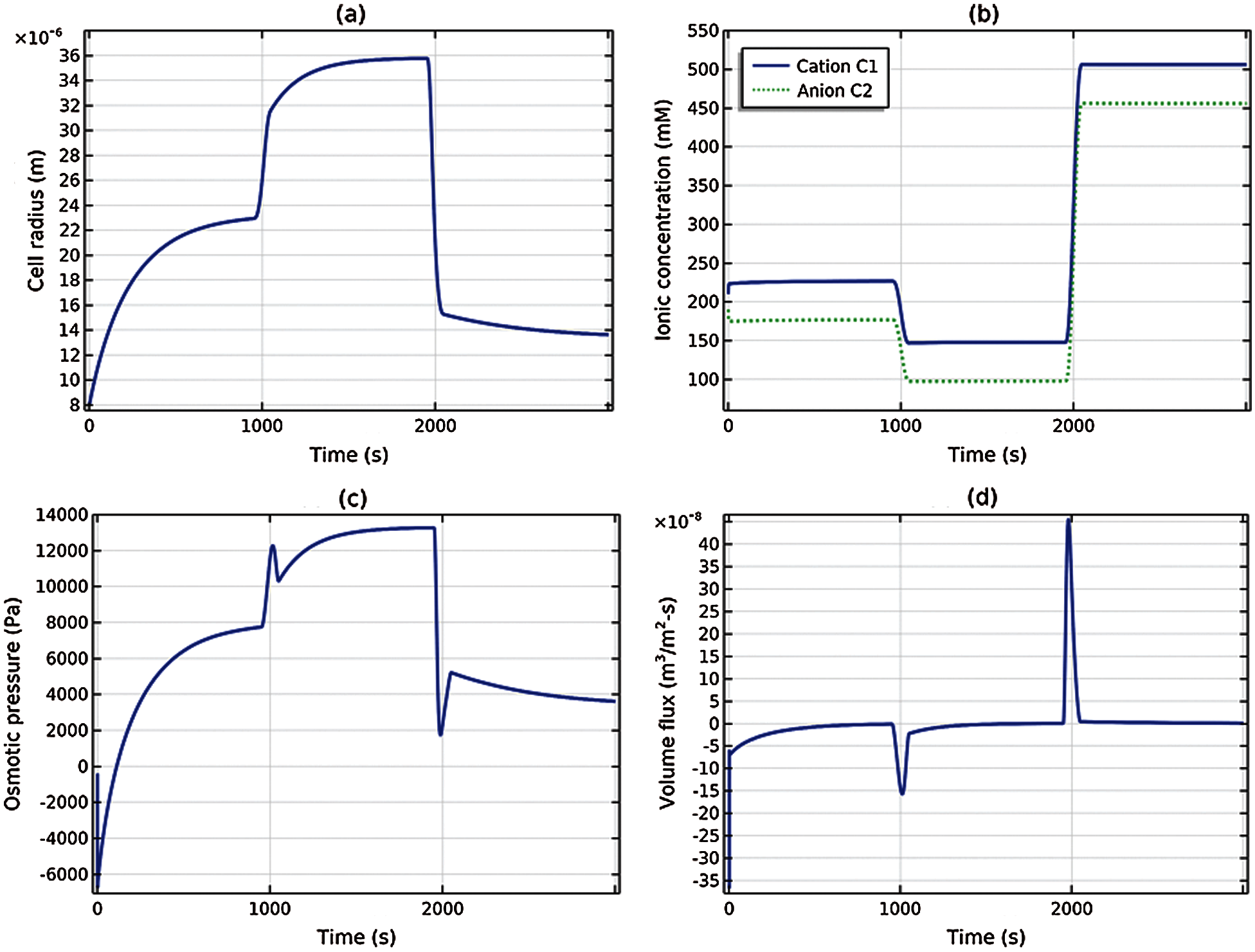
Figure 4: Transient response of the cell, with only passive ionic transport, to the osmotic shock loading given by (65): (a) Cell radius, (b) cation and anion concentrations, (c) osmotic pressure, (d) volume flux
6.3 Active Ionic Transport with Osmotic Shock
Using the same arbitrary initial values noted in the preceding subsection, 1000 s is allowed for the system to reach near steady state. The cell is then subject to a single hypotonic shock at t = 1000 s according to
Active cation (Na+) pumping is initiated when the membrane tension τ = τcrit. For this simulation, we assumed
with active flux magnitude
Fig. 5 compares two solutions. The solid (blue) curves correspond to passive transport only
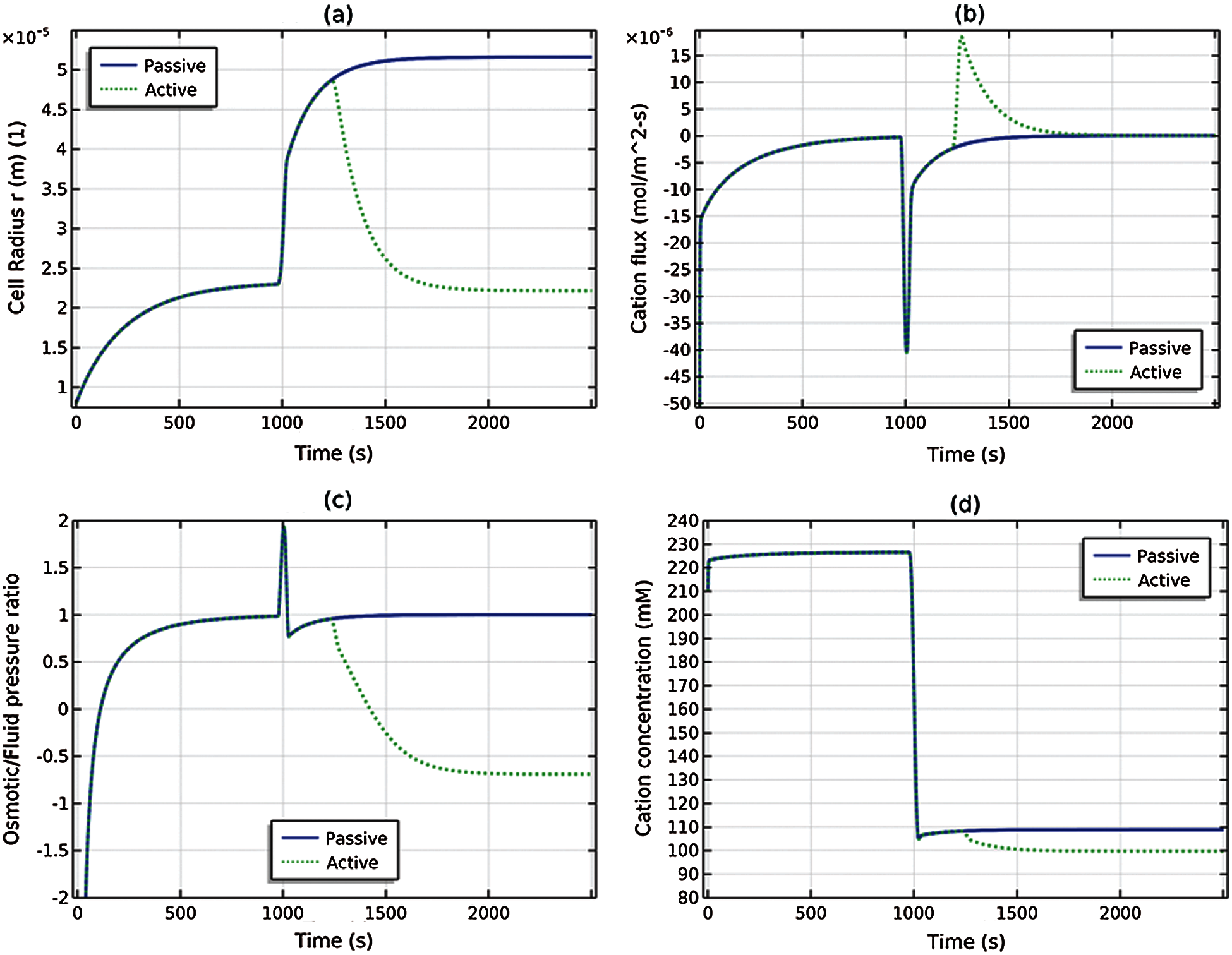
Figure 5: The cell is subjected to a hypotonic shock at t = 1000 s and two solutions are depicted. The solid (blue) curve shows the passive transport case and the dotted (green) curves show the active transport case: (a) cell radius r(t), (b) cation flux J1(t), (c) ratio of osmotic and fluid pressure ΔP(t)/ΔΠ(t), and (d) cation concentration C1(t)
Fig. 6a shows a side-by-side comparison of the osmotic and fluid pressures for the passive transport solution; Fig. 6b shows the same comparison for the active transport solution. It is seen from Fig. 6a that at steady (equilibrium) state (t = 1000 and t = 3000 s), the fluid and osmotic pressures coincide (as necessary, see (35)), whereas in Fig. 6b the osmotic and fluid pressures deviate significantly at steady (nonequilibrium) state (t = 3000 s) due to the active cation transport. The difference between the osmotic and fluid pressures is the active pressure component ΔPa = ΔP − ΔΠ given by (48). At steady state (t = 3000 s) and using (55),
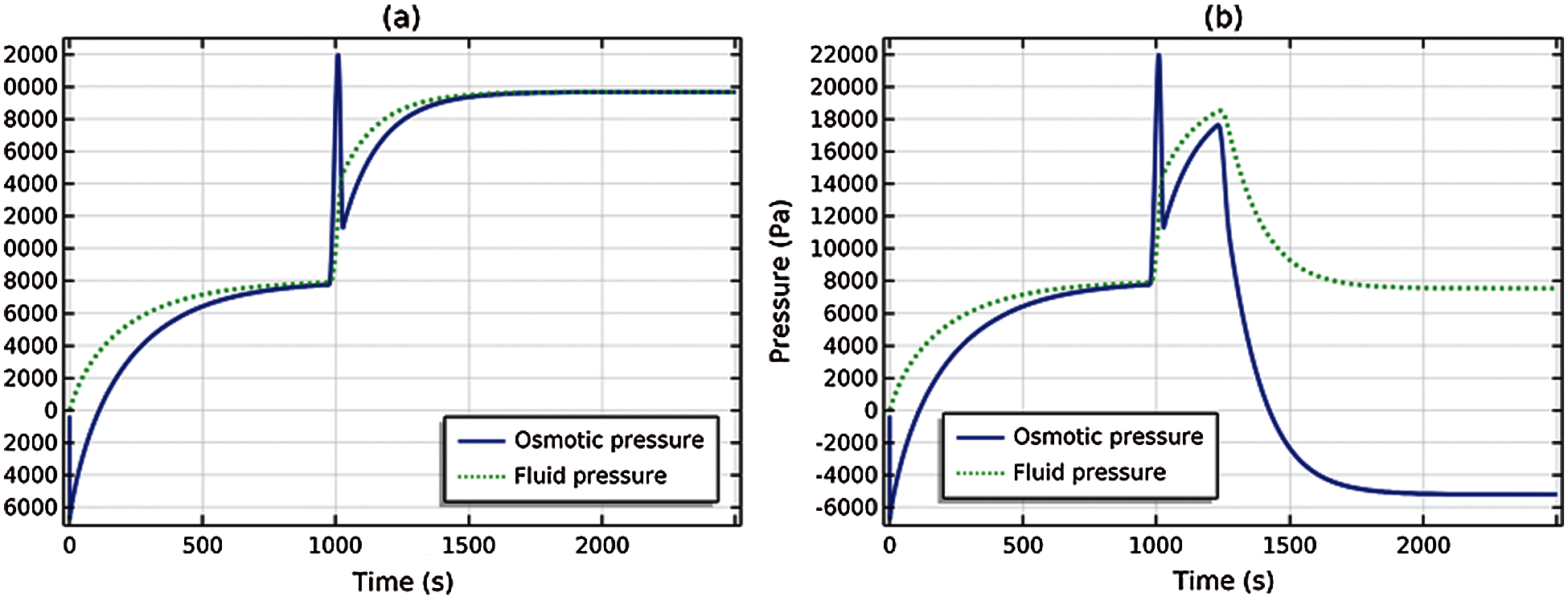
Figure 6: Comparison of osmotic and fluid pressures. (a) Passive transport solution, (b) Active cation transport solution
The results of a parametric study of the osmotic shock problem (67) is provided in Fig. 7. Figs. 7a and 7b show the cell radius and osmotic pressure for
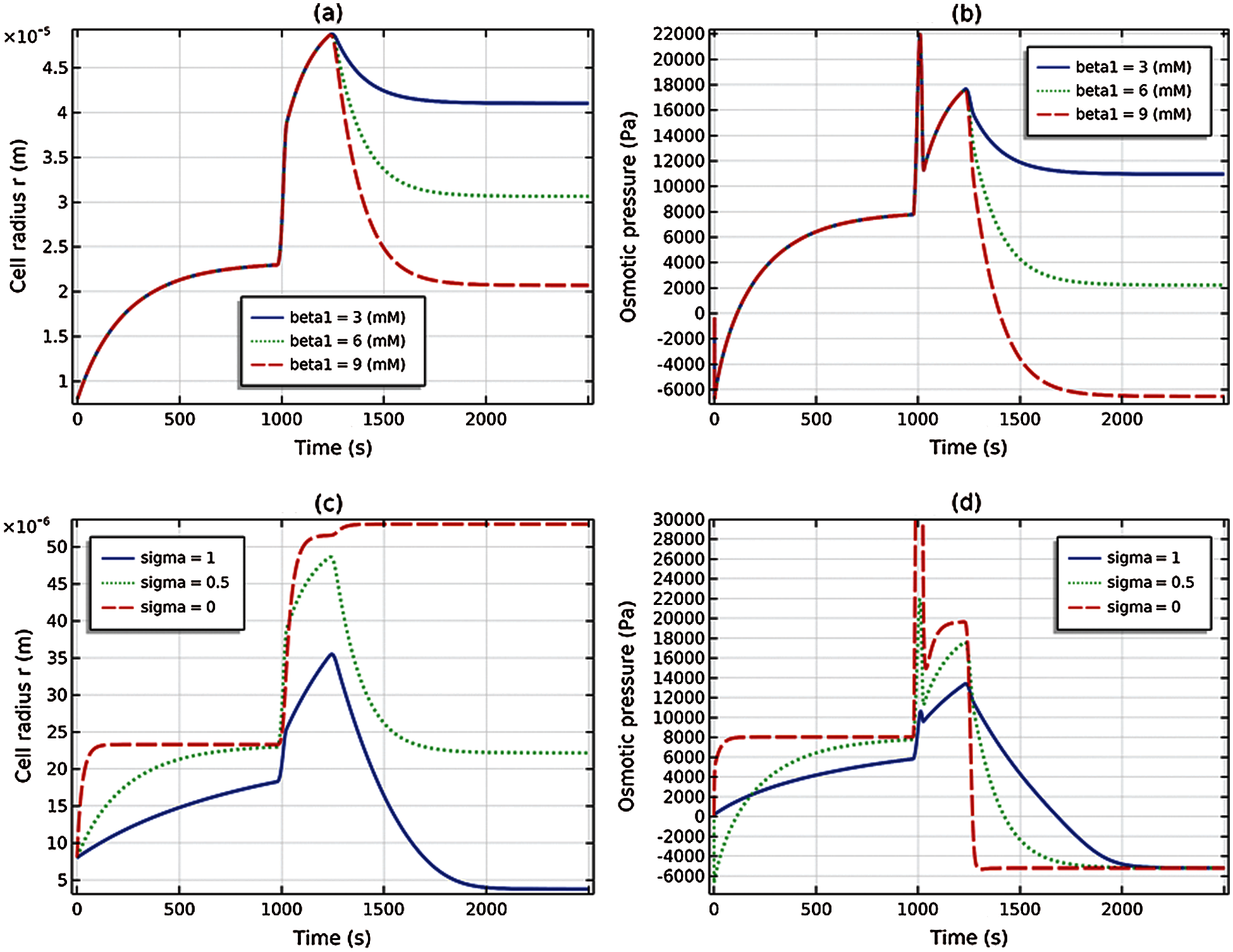
Figure 7: Parametric studies. (a) and (b) Active cation flux magnitude
The presented development of the phenomenological equations for passive and active transport for cell membranes follows standard lines for nonequilibrium thermodynamics. But an important step for electrolytes with impermeant charged macromolecules included the incorporation of fixed charge through the condition of electroneutrality. For passive transport of ionic solutions and at steady state, the theory recovers the Donnan equilibrium and predicts agreement of the osmotic and fluid pressure differences across the membrane such that ΔP = ΔΠ. When active ion transport processes are present, the theory predicts that an active fluid pressure component ΔPa arises as a direct result of the active fluxes. This term enters into the balance of fluid and osmotic pressure such that
The model evaluated the membrane potential Δψ by using the assumption that it is not changing rapidly with time. This led to the condition that the sum of the active and passive currents vanish
Following presentation of the general theory for an arbitrary number of ionic species, the theory was adapted to model the pump-leak mechanism (PLM) in a eukaryotic cell model. The cell was modeled as a spherical elastic shell with area elasticity but no attempt was made to model the dependence of the modulus on the cell volume or other possible structural characteristics. However, the cell cortex—lipid membrane system so modeled was able to develop tension and support fluid pressure—allowing both fluid and osmotic pressures to be modeled and studied for both passive and active transport. The intracellular and extracellular media were taken to be simple binary electrolytes containing Na+ and Cl− ions. Trapped (nonpermeant) charged macromolecules were included in the intracellular medium but no attempt was made to model the “excluded volume” effects associated with the molecular volumes (this is essentially treated by specification of an effective charge concentration Cf). Temporal changes in concentrations took account of membrane transport processes and the dynamically evolving cell area and volume. Active ion transport results were reported using an Na+ pump to stabilize cell volume against osmotic forces that would otherwise drive water into the cell.
The PLM mechanism is clearly demonstrated in Fig. 8. The problem solved is that described in Section 6.3; the cell is subjected to a hypotonic shock at time t = 1000 s, at which time it begins to swell as indicated by the increasing cell radius and the jump in water inflow (negative values of Jv). Initiation of Na+ pumping occurs when the membrane tension reaches a critical value, at time t ≈ 1,250 s. The cell volume now starts to decrease and approach a steady state. The direct correlation of Na+ active flux with water outflow (positive values of Jv) is apparent in the figure and suggests capture of the PLM.
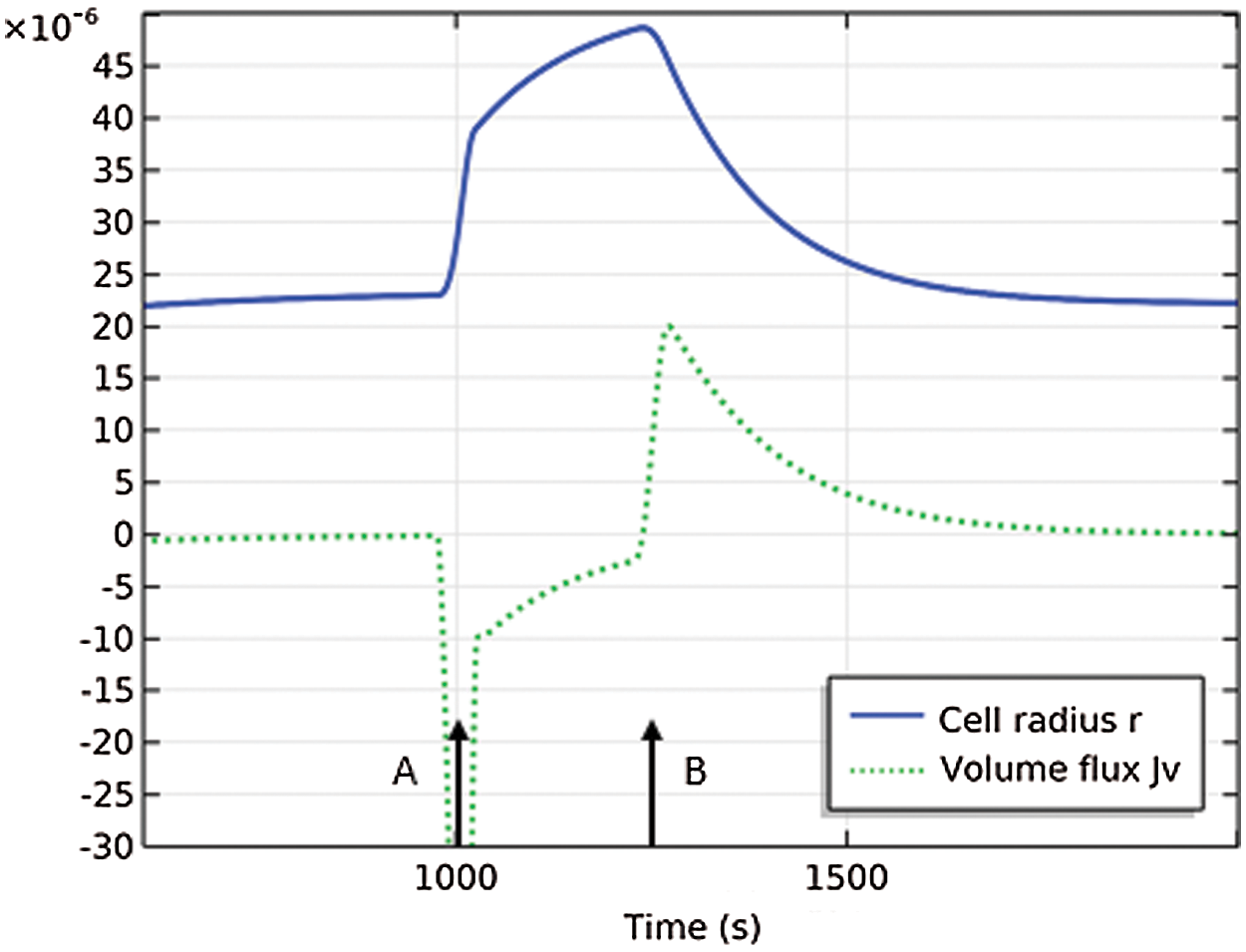
Figure 8: Cell undergoes hypotonic shock at time A and initiates active cation pumping at time B. The cell radius is r(m) and the volume flux is Jv (mol/m2 − s) and has been scaled by 120. At time A, the cell begins to swell by water inflow. At time B, the cell begins to deswell by water outflow
Previous models of the PLM, presented in the field of mathematical physiology have been successful in demonstrating the key aspects of the mechanism [2,3,5,19]. However these models cannot exhibit a stable Donnan equilibrium, in contrast to the current study. Cells cannot be at a stable Donnan equilibrium unless they can develop and sustain a transmembrane fluid pressure. For example, in prior models, if ions are not actively pumped, the cell volume will increase without limit and no steady state will be found. The current model develops fluid pressure and arrives at steady state according to the hydraulic conductivity (see, for example, Fig. 5a). It has been claimed that cells cannot be at a stable Donnan equilibrium, but this is true only if they cannot support internal fluid pressure [20]. The current model shows clearly that in the case of passive ion transport, Donnan equilibrium can be achieved using realistic values of the cell membrane elasticity.
In practice, the primary contributors to intracellular and extracellular tonicity are Na+, K+ and Cl− , all of which are permeable solutes. The Na+ pump (Na+ − K+ ATPase) actively drives Na+ out and K+ in [5,21]. Ion channels and ion pumps drive the physiological system, aquaporins (water channels) likewise modulate the hydraulic conductivity of the cell membrane. These essential features underlying the PLM can be modeled within the presented framework, based on experimental evidence, and would provide a more complete representation of the PLM.
1Since we have no knowledge of how Ck varies across the membrane, a good approximation is:
The Heaviside functions H(t − tshock) were smoothed over a transition zone of 100 s in order to be more physically realistic.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declares that they have no conflicts of interest to report regarding the present study.
1. Hoffmann, E., Lambert, I., Pedersen, S. (2009). Physiology of cell volume regulation in vertebrates. Physiological Reviews, 89(1), 193–277. DOI 10.1152/physrev.00037.2007. [Google Scholar] [CrossRef]
2. Armstrong, C. (2003). The Na/K pump, Cl ion, and osmotic stabilization of cells. Proceedings of the National Academy of Sciences, 100(10), 6257–6262. DOI 10.1073/pnas.0931278100. [Google Scholar] [CrossRef]
3. Kay, A. (2017). How cells can control their size by pumping ions. Frontiers in Cell and Developmental Biology, 5(41), 1–14. DOI 10.3389/fcell.2017.00041. [Google Scholar] [CrossRef]
4. Kay, A., Blaustein, M. (2009). Evolution of our understanding of cell volume regulation by the pump-leak mechanism. Journal of General Physiology, 151(4), 407–416. DOI 10.1085/jgp.201812274. [Google Scholar] [CrossRef]
5. Keener, J., Sneyd, J. (2009). Mathematical physiology I: Cellular physiology. Second edition. New York: Springer. [Google Scholar]
6. Stewart, M., Helenius, J., Toyoda, Y., Ramanathan, S., Muller, D. et al. (2011). Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature, 469, 226–230. DOI 10.1038/nature09642. [Google Scholar] [CrossRef]
7. Jiang, H., Sun, S. (2013). Cellular pressure and volume regulation and implications for cell mechanics. Biophysical Journal, 105(3), 609–619. DOI 10.1016/j.bpj.2013.06.021. [Google Scholar] [CrossRef]
8. Charras, G., Yarrow, J., Horton, M., Mahadevan, L., Mitchison, T. (2005). Non-equilibration of hydrostatic pressure in blebbing cells. Nature, 435, 365–369. DOI 10.1038/nature03550. [Google Scholar] [CrossRef]
9. Fraser, J., Middlebrook, C., Usher-Smith, J., Schwiening, C., Huang, C. (2005). The effect of intracellular acidification on the relationship between cell volume and membrane potential in amphibian skeletal muscle. The Journal of Physiology, 563, 745–764. DOI 10.1113/jphysiol.2004.079657. [Google Scholar] [CrossRef]
10. Dawson, D., Liu, X. (2009). Osmoregulation: Some principles of water and solute transport. In: D. Evans (ed.Osmotic and ionic regulation: Cells and animals. pp. 1–35. Boca Raton, FL: CRC Press. [Google Scholar]
11. Kedem, O., Katchalsky, A. (1958). Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochimica et Biophysica Acta, 27(2), 229–246. DOI 10.1016/0006-3002(58)90330-5. [Google Scholar] [CrossRef]
12. Friedman, M. (2008). Principles and models of biological transport. Second edition. New York: Springer. [Google Scholar]
13. Kedem, O., Katchalsky, A. (1963). Permeability of composite membranes. Part 1. Electric current, volume flow and flow of solute through membranes. Transactions of the Faraday Society, 59, 1918–1930. DOI 10.1039/TF9635901918. [Google Scholar] [CrossRef]
14. Li, L. (2004). Transport of multicomponent ionic solutions in membrane systems. Philosophical Magazine Letters, 84(9), 593–599. DOI 10.1080/09500830512331325767. [Google Scholar] [CrossRef]
15. Cheng, X., Pinsky, P. (2015). The balance of fluid and osmotic pressure across active biological membranes with application to the corneal endothelium. PLoS One, 10(12). DOI 10.1371/journal.pone.0145422. [Google Scholar] [CrossRef]
16. Demirel, Y., Sandler, S. (2002). Thermodynamics and bioenergetics. Biophysical Chemistry, 97(2), 87–111. DOI 10.1016/S0301-4622(02)00069-8. [Google Scholar] [CrossRef]
17. Schultz, S. (1980). Basic principles of membrane transport, New York: Cambridge University Press. [Google Scholar]
18. Adar, R., Safran, S. (2020). Active volume regulation in adhered cells. Proceedings of the National Academy of Sciences, 117(11), 5604–5609. [Google Scholar]
19. Mori, Y. (2012). Mathematical properties of pump-leak models of cell volume control and electrolyte balance. Journal of Mathematical Biology, 65, 875–918. DOI 10.1007/s00285-011-0483-8. [Google Scholar] [CrossRef]
20. Sperelakis, N., (2012). Gibbs-donnan equilibrium potentials. In: N. Sperelakis (Ed.Cell physiology sourcebook. Third edition, pp. 147–151. New York: Academic Press. [Google Scholar]
21. Stein, W. (1995). The sodium pump in the evolution of animal cells. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 349, 263–269. DOI 10.1098/rstb.1995.0112. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |