
 | Computer Modeling in Engineering & Sciences |  |
DOI: 10.32604/cmes.2021.016269
ARTICLE
A Computational Study on Lateral Flight Stability of the Cranefly in Hover
1School of Electromechanical and Automotive Engineering, Yantai University, Yantai, 264005, China
2School of Civil Engineering, Yantai University, Yantai, 264005, China
*Corresponding Author: Xiaolei Mou. Email: xiaoleimou@126.com
Received: 22 February 2021; Accepted: 07 May 2021
Abstract: The dynamic flight stability of hovering insects includes the longitudinal and lateral motion. Research results have shown that for the majority of hovering insects the same longitudinal natural modes are identified and the hovering flight in longitudinal is unstable. However, in lateral, the modal structure for hovering insects could be different and the stability property of lateral disturbance motion is not as robust as that of longitudinal motion. The cranefly possesses larger aspect ratio and lower Reynolds number, and such differences in morphology and kinematics may make the lateral dynamic stability different. In this paper, the lateral flight stability of the cranefly in hover is investigated by numerical simulation. Firstly, the stability derivatives are acquired by solving the incompressible Navier–Stokes equations. Subsequently, the dynamic stability characteristics are checked by analyzing the eigenvalues and eigenvectors of the linearized system. Computational results indicate that the lateral dynamic modal structure of cranefly is different from most other insects, consisting of three natural modes, and the weakly oscillatory mode illustrates the hovering lateral flight is nearly neutral. This neutral stability is mainly caused by the negative derivative of roll-moment vs. sideslip-velocity, which can be attributed to the weaker ‘changing-LEV-axial-velocity’ effect. These results suggest that insects in nature may exhibit different dynamic stabilities with different morphological and kinematic parameters, which should be considered in the designs of flapping wing air vehicles.
Keywords: Flapping flight; cranefly; lateral flight stability; natural modes of motion; computational fluid dynamics
Since the insects flying in nature are disturbed by the surroundings all the time, the problems that how the insects change the flight attitude with the influence of external disturbances, how they maintain a stable flight attitude and speed, and how they achieve the inspired performances, such as an abrupt stop, turn, fast acceleration, should be investigated. To reveal the flight dynamic stability and control mechanism of insects, extensive studies on these above problems have been conducted in recent decades, which are available in the literature reviews [1,2]. Considering that the flight dynamics of a flapping-wing flier is inherently complex, and only the dynamic stability is thoroughly researched can the control problem be adequately understood, many investigations mainly focused on dynamic flight stability to examine the stability derivatives, dynamic modal structure and quantitative stability properties [3–13].
In the above investigations [3–13], with the averaged model widely employed, the flapping-wing fliers are approximately treated as rigid bodies with six degrees of freedom (6-DOF), the equations of motion of which are similar to the conventional aircraft; then using the linear theory, the disturbed flight in the longitudinal and lateral direction are treated separately after decoupling, and in turn, the stability of the flapping-wing systems could be checked by techniques of eigenvalue and eigenvector analyses. The disturbed flight includes longitudinal and lateral motion, and only both longitudinal and lateral dynamics are figured out can the stability characteristics of insects be known.
During the past two decades, many research work, employing different aerodynamic models, the CFD method or the quasi-steady model, have been concentrated on the longitudinal dynamic stability [4–6,9,10,12,13] for several hovering insects (e.g., bumblebee, hoverfly, dronefly, cranefly, fruitfly, stalk-eyed fly, hawkmoth and mosquito) and the literatures reported that three longitudinal natural modes (two stable subsidence modes and one unstable oscillatory mode) in hover were identified, thus the hovering flight in longitudinal was unstable. In addition, the conclusions of longitudinal dynamic stability mentioned above were consistent, although the mass of research objects ranged from 1 to 1648 mg and wing-beat frequency from 26 to 800 Hz [2,13,14]. With the wider range of weight and wing-beat frequency, the longitudinal dynamic stability characteristics mentioned above may represent those of the majority of insects.
Researches on lateral dynamic stability of several hovering insects were carried out gradually, and all the literatures pointed out the lateral disturbed flight was composed of three natural modes of motion. However, the modal structure of lateral motion for different hovering insects is not exactly the same. The works in Zhang et al. [8] and Xu et al. [11,15] reported that the lateral motion of hovering insects (dronefly, hoverfly and bumblebee) was unstable, due to the divergence mode; and works in Faruque et al. [7], Cheng et al. [9] and Kim et al. [12] showed that all the natural modes of hovering insects (fruitfly, hawkmoth, stalk-eyed fly and bumblebee) in lateral were stable. Hereafter, the study on the lateral dynamic stability of hovering honeybee [16] pointed that the lateral disturbed motion consisted of a nearly neutrally stable oscillatory mode and two stable subsidence modes, hence the flight was neutrally stable. The difference in the modal structure of lateral motion could be attributed to the different sign of the derivative of roll moment vs. sideslip velocity [11,12,16] (hereafter called as roll-moment/sideslip-velocity derivative). Contributions on the generation of the roll-moment/sideslip-velocity derivative included two flow effects: ‘changing-relative-velocity’ effect and ‘changing-LEV-axial-velocity’ effect (hereafter abbreviated as CRV-effect and CLV-effect), and the relative sizes of which determined the sign of the derivative [11,15,16].
As noted above, the researches on longitudinal dynamics in hover cover many kinds of insects, additionally with various aspect ratios (AR, the ratio of wing length to mean chord length), and the longitudinal dynamic stability characteristics mentioned are similar. However, the roll-moment/sideslip-velocity derivative could be affected by certain parameters, e.g., the location of the CG (the center of gravity), the stroke plane angle [15,16]. In addition, the recent study [17] pointed out the wing-wing interaction had influence on the roll-moment/sideslip-velocity derivative and the contralateral wing stabilized the hovering hawkmoth in lateral. In other words, the lateral stability, which may have different modal structure, is not as robust as that of longitudinal. Furthermore, the insects considered in the studies of lateral dynamic stability are mostly with moderate aspect ratios (AR = 2–4), excluding the insects with a relatively large aspect ratio. Researches have showed that the change in AR have the influence on the flow structure of the rotating and flapping wings [18–20] and hence the aerodynamic forces acting on the wings. This situation may have impact on the aerodynamics of insects with a relatively large aspect ratio in disturbed flight, especially the roll-moment/sideslip-velocity derivative that can result in the lateral stability changing. For some insects, even without the wing-wing interaction, the CLV-effect may decrease due to a relatively large aspect ratio, and the relative size of roll moments produced by the two flow effects involved above which have opposite signs accordingly changed, and hence the negative roll-moment/sideslip-velocity derivative and the flight stability would become different. Recently, Liu et al. [13] have discussed the dynamic stability of mosquitoes in hover of which the aspect ratio is indeed relatively large (AR = 5), and the results showed that the dynamic modal structure of mosquitoes is similar to that of other insects, such as bumblebee and hoverfly. However, the mosquitos used different mechanisms to generate aerodynamic forces due to the rather short stroke amplitude [14], while much more insects considered above mainly use the same delayed-stall mechanism. Among the insects studied above, the cranefly has a relatively large aspect ratio (AR = 5.5) and also use the delayed-stall mechanism. From the foregoing discussion, it is meaningful to investigate the lateral dynamic stability of hovering cranefly to see whether or not it has similar dimensionless stability derivatives and dynamic modal structure with other insects.
In this paper, taking a model cranefly in hover as the research object, numerical research on its lateral dynamic stability is conducted. First, the incompressible Navier-Stokes equations are solved to acquire the stability derivatives, and then the eigenvalues and eigenvectors of the system matrix are derived to characterize the lateral dynamics. The analysis results verify the previous hypothesis: the roll-moment/sideslip-velocity derivative of the model cranefly is negative and a different dynamic modal structure from most other insects is identified.
In this study, the cranefly Tipula obsoleta is conducted as the research target based on measurement data [21], the total weight of the cranefly
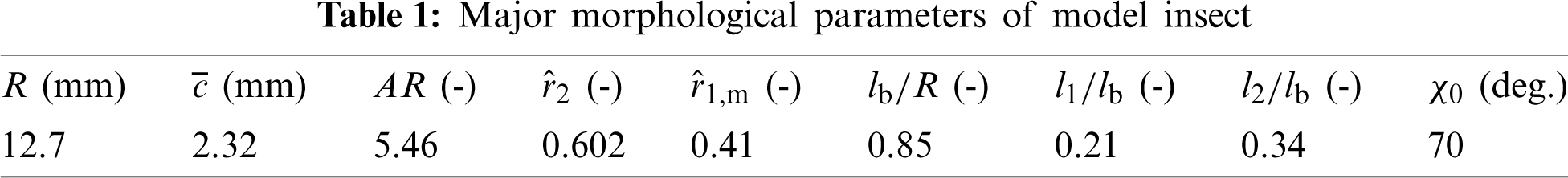
In order to describe the definitions of the wing and body kinematics, two coordinate systems are introduced here: a right-handed body-fixed frame (
Figure 1: Schematics of the kinematics of model insect (A) the right-handed body-fixed frame, (B) the wing-fixed frame
The origin of the wing-fixed frame
When the wing flaps to its extreme position, the stroke positional angle
where
All the kinematic parameters needed to describe the motions of body and wing are detailed in Tab. 2 and determined from experimental data by Ellington [22], excluding

Referring to the earlier studies [3,4,8], the averaged model is applied in the present study. Provided the model insect presents a relatively high wingbeat frequency, it is assumed that the flapping flier can be treated like a 6-DOF rigid body (three in translation and three in rotation) and use the mean aerodynamic forces and moments through the flapping period to represent the role of wings in aerodynamics. Therefore, the motions of model insect are expressed by the equations of motion of rigid body, commonly used for the airplane or helicopter.
For the convenience of describing the movements of model insect, the right-handed frame of reference (
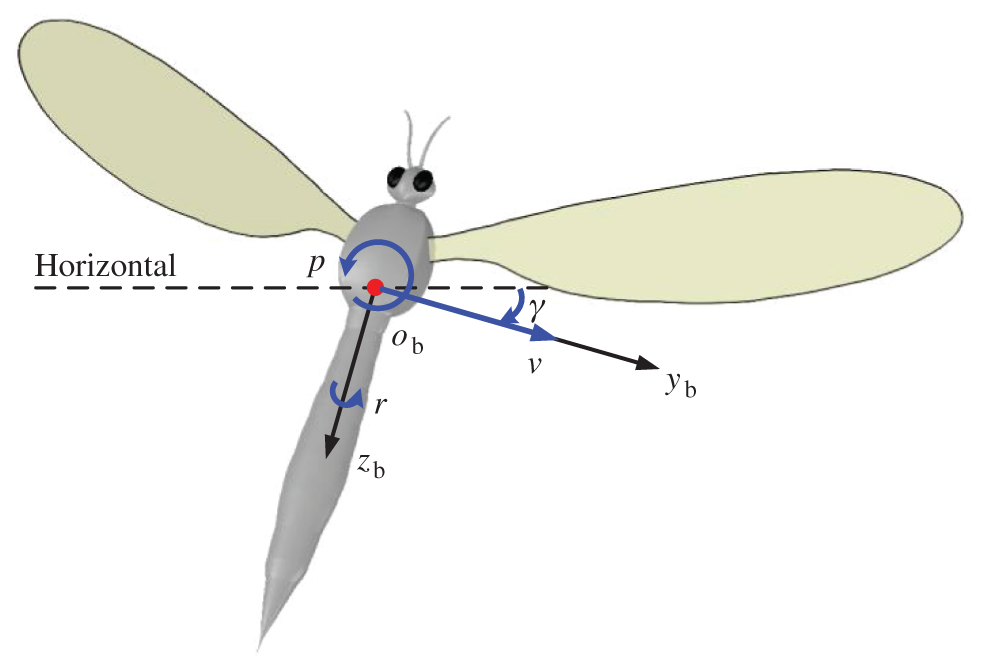
Figure 2: Definition of the lateral state variables
For the convenience in analyzing, the components of average aerodynamic forces through the flapping period are recorded as
where the matrix A is the stability system matrix, the prefix
In the matrix A, the symbols (
The moments of inertia about
2.3 Flow Computation and Grid Resolution Test
By solving the Navier–Stokes (N-S) equations, the equilibrium flight condition can be obtained, and hence the determination of stability derivatives. In this study, the identical numerical calculating method which Sun et al. [23] had given the detailed description in their work is adopted for simulation and based on the artificial compressibility approach [24]. The earlier research result [25] showed that the corrugation and deformation of wings in the flapping have little effects on aerodynamic forces, and thus the wing of model insect can be dealt with as a rigid plate by neglecting the corrugation and deformation. By comparison with wings, the body has very low velocity near hovering and thus the aerodynamic forces and moments generated by the body are negligible; besides, the aerodynamic interference between the wings and body is so weak that it can be neglected, as well as the interference between the right and left wings [26]. Thus, the flow around the right and left wing can be calculated separately.
In this study, the planform of wing model used here is taken from the scanned image of the real wing [21] (Fig. 3, the solid line with an arrow indicates the pitching rotation axis). The numerical calculation program used here has been validated and used many times in previous studies (e.g., [8,11,23]). Reynolds number (
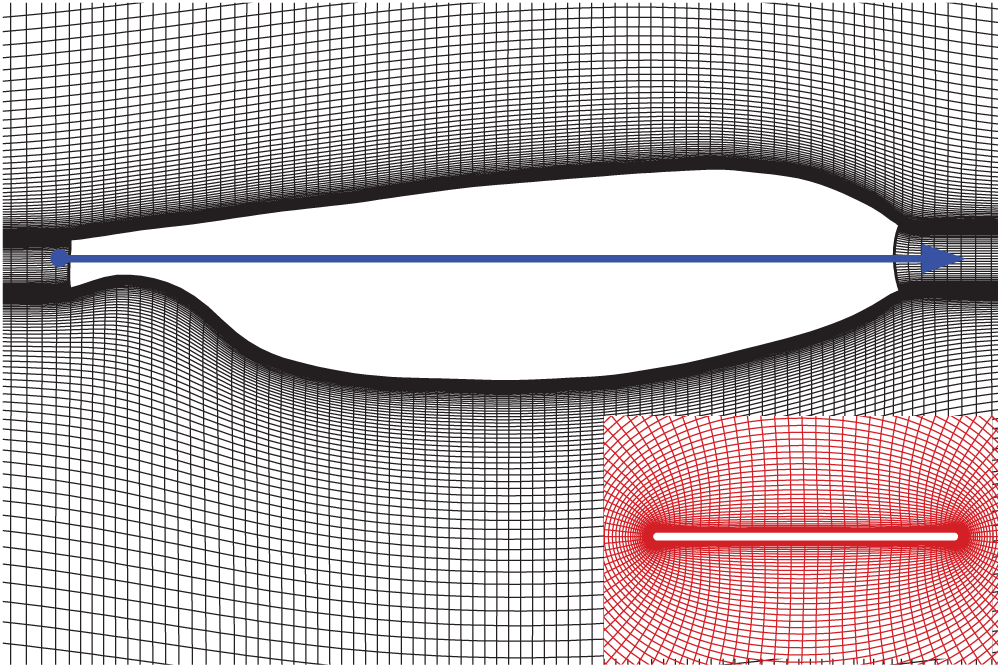
Figure 3: The planform of the wing and portions of the grid. The inset is the sectional plane at the radius of gyration (
In order to make sure that the flow simulations are grid independent, three grids are used herein to conduct the grid resolution test before the simulations. The size of Grid 1 is 25 × 27 × 34 (in the circumferential direction, the radial direction and the spanwise direction, respectively) with the first layer spacing of
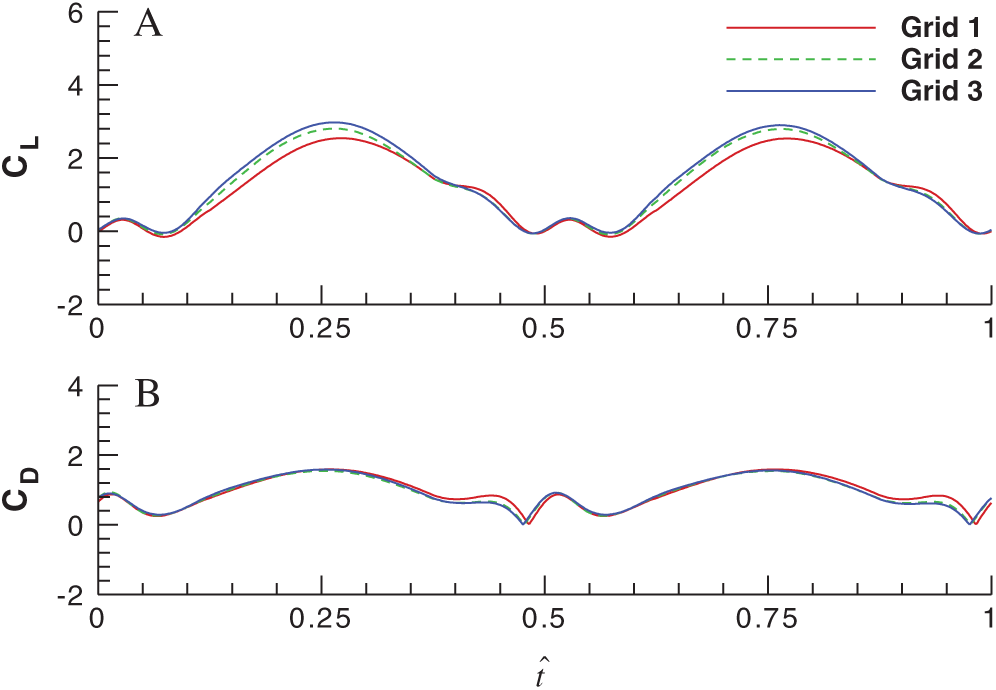
Figure 4: Effects of grid density on the lift and drag coefficients over a single flapping cycle (A) for the lift coefficient and (B) for the drag coefficient
2.4 Calculation of the Stability Derivatives
As aforementioned,
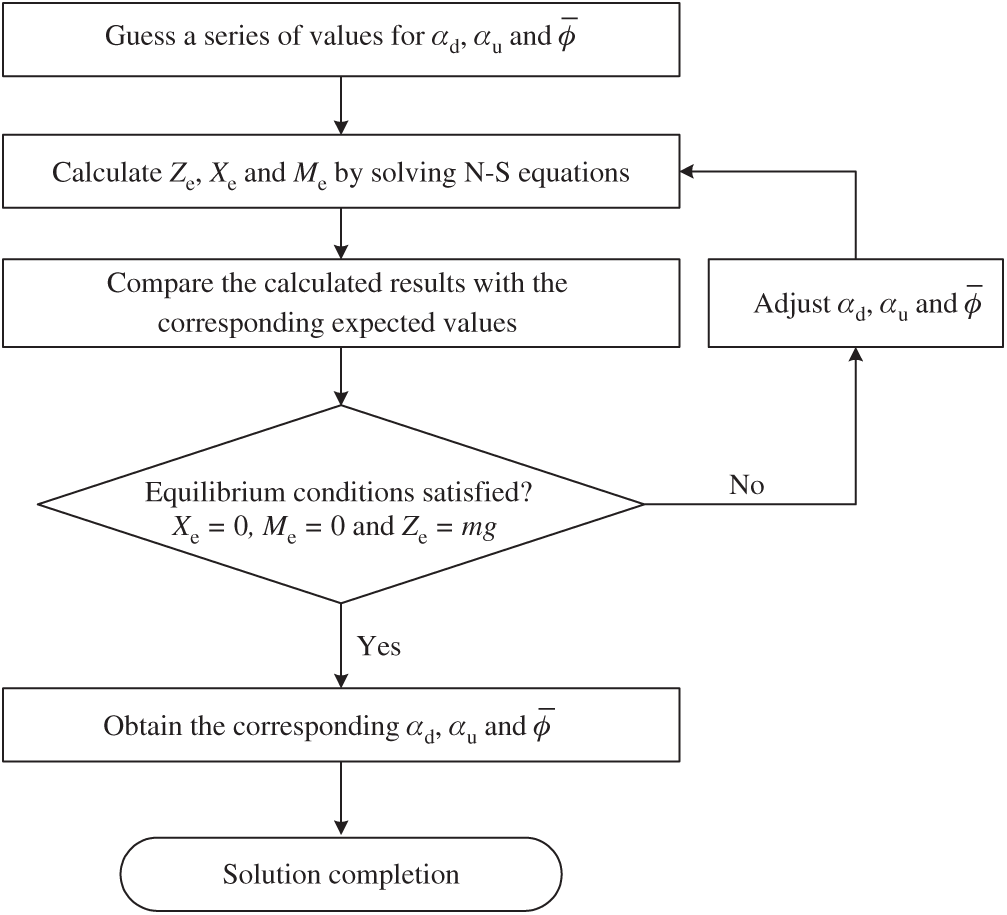
Figure 5: The solution process of equilibrium conditions achievements
Once the equilibrium flight is determined, we can use it as the reference flight state to acquire the stability derivatives. Adopting the same method as taken in the previous studies [8,11], three series of flow computations are conducted. For each series, only one of the body velocities varies near zero whilst the others keep the reference values unchanged. Take the v-series as an example, the lateral translational velocity
Once the derivatives are calculated, all the data needed in the system matrix A in Eq. (3) are determined. Adopting the same eigenvalues and eigenvectors analysis method in previous studies [4,8,11], the flight stability characteristics of the system are acquired from the linear small disturbance equations.
As described above, on the basis of the equilibrium flight of model cranefly, the body velocities v, p and r vary around zero respectively and the corresponding aerodynamic forces and moments are obtained, which are shown in Fig. 6.
According to the results of Tab. 3, the lateral stability derivatives
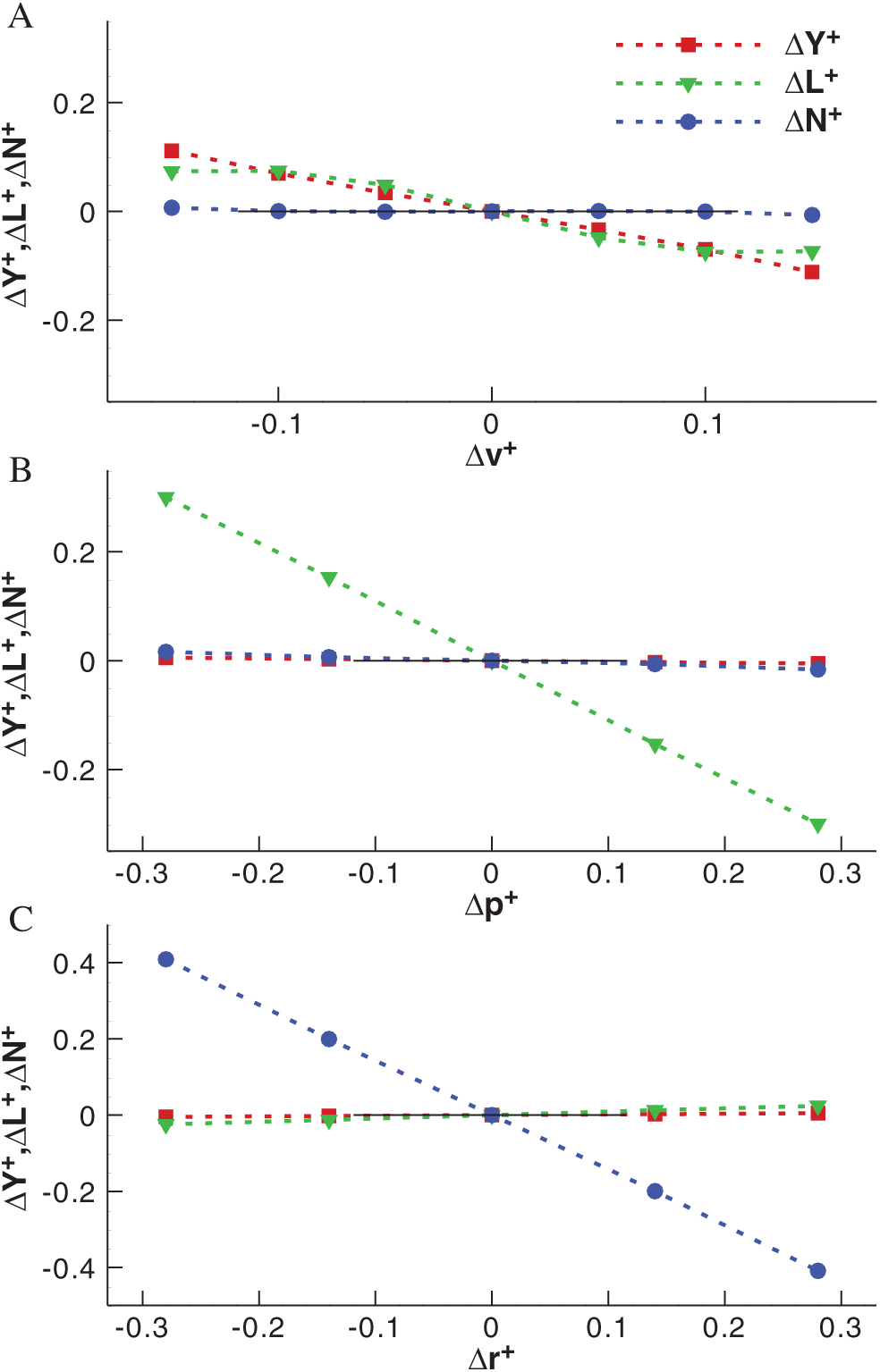
Figure 6: The variations of aerodynamic forces and moments with body velocities for model cranefly (A) for the v-series, (B) for the p-series and (C) for the r-series
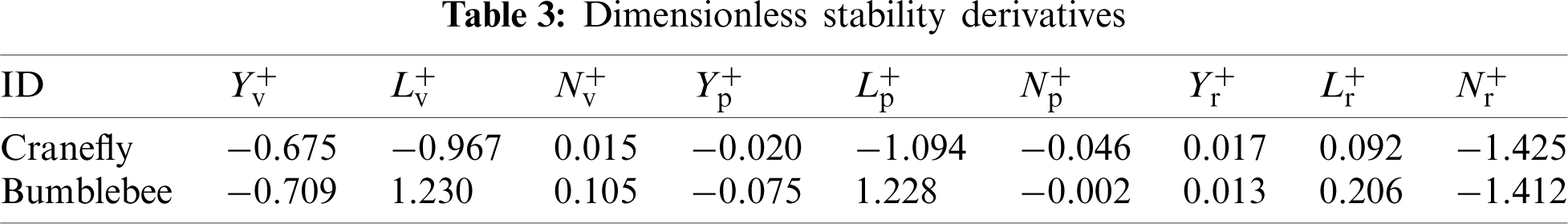
3.2 Generation Mechanism of Stability Derivatives
As stated earlier, the generation of the mainly stability derivatives,
To illuminate the reason why the derivative
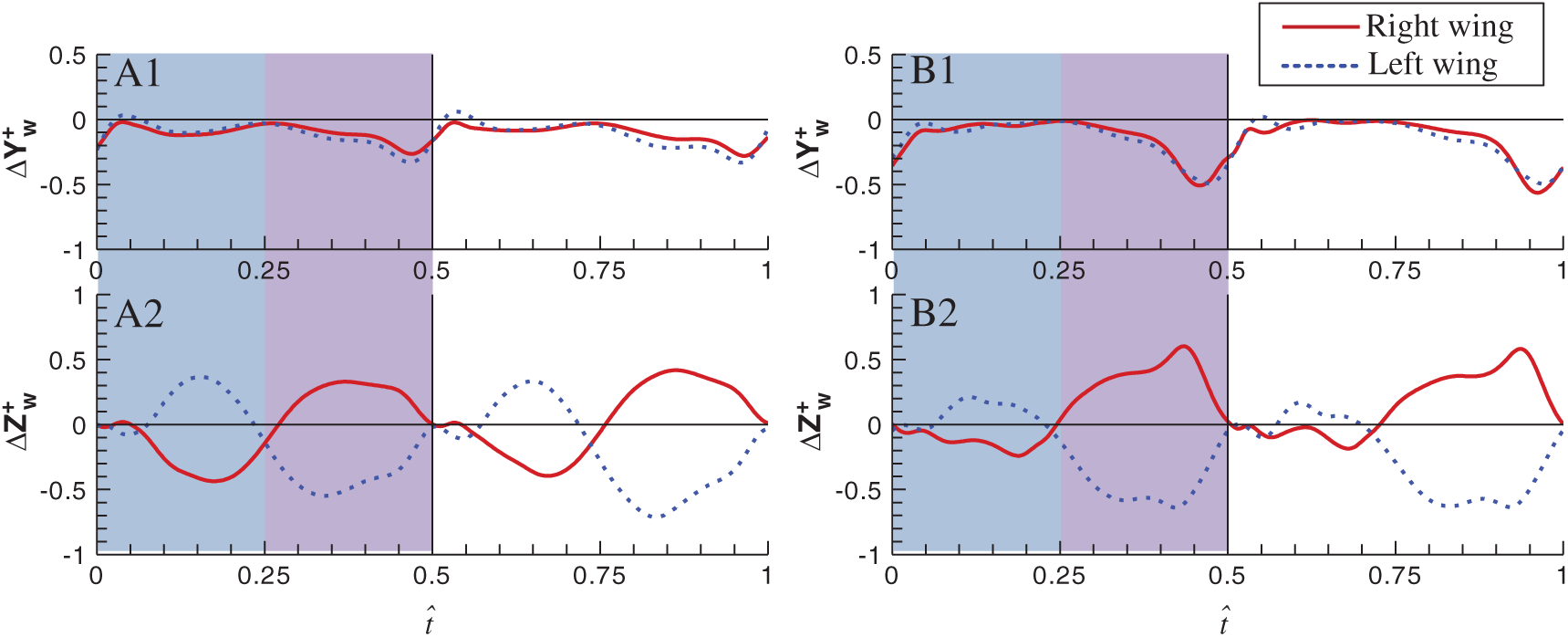
Figure 7: Time-variation of
Figure 8: Schematics of roll moments produced by the force couple during the downstroke for model cranefly and bumblebee (A) for cranefly, (B) for bumblebee; the pale blue for the first part of the downstroke and the lavender for the latter part of the downstroke
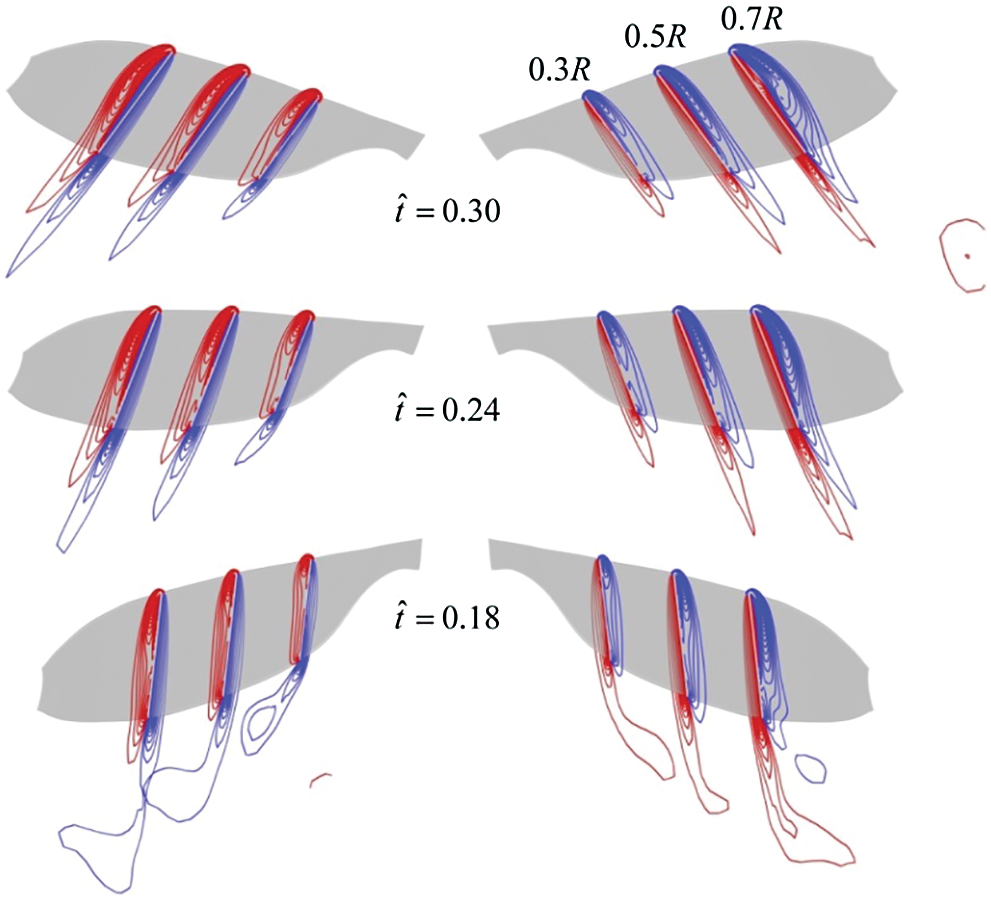
Figure 9: Spanwise vorticity plots at wing sections (0.3R, 0.5R, 0.7R from wing root, respectively) when the insect moves to the right at
Next, the CRV-effect and CLV-effect of model cranefly and those of model bumblebee [11] will be quantitatively compared. Still take insects move to the right at
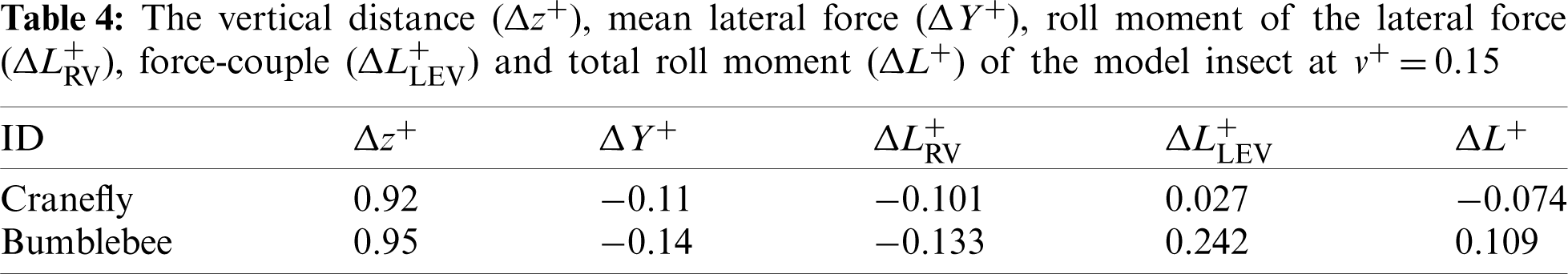
According to the results of analysis above, under the side wind, for the model cranefly, the CLV-effect is weaker than the CRV-effect, meaning the positive contribution given by the CLV-effect is small; thus, the net roll moment is negative, and hence the negative derivative
In this section, the lateral stability properties of the hovering model cranefly are investigated here with the stability derivatives and morphological data given above. Based on the averaging theorem, the dynamic stability could be examined via the eigenvalues and eigenvectors of its matrix A. For the model cranefly, the results of eigenvalues and eigenvectors showed in Tab. 5 indicate three lateral motion modes.
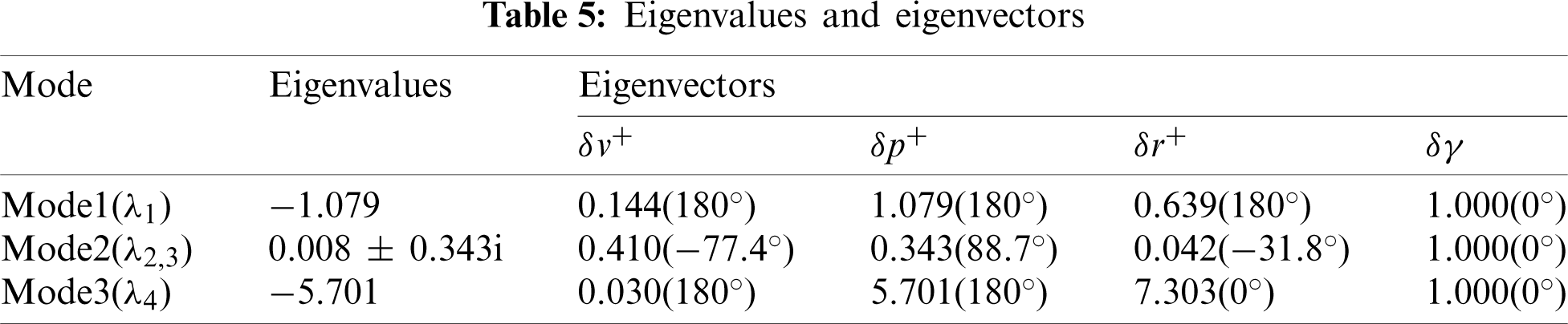
Mode 1 corresponds to a stable subsidence mode with a negative real eigenvalue (
It can be seen from the above analysis that, comparing with the hovering bumblebee model [11], the modal structure of cranefly is different. Due to the weakly unstable mode, the lateral dynamics of hovering cranefly model is close to neutrally stable. Xu et al. [16] reported that the weakly unstable mode is chiefly due to the derivative
3.4 Discussion on the Stability Characterization
In the literatures, different aerodynamic models with different fidelity levels, the quasi-steady model [7,9,12,17] and the CFD method [8,11,13,16], are commonly applied to acquire the stability derivatives and identify the dynamic stability for various hovering insects in the lateral direction. This section focuses on comparisons of the lateral stability characteristics in this paper and some previous studies.
For the quasi-steady aerodynamic models, the lateral motion of hovering insects is stable due to the negative roll moment derivative [7,9,12,17]. Herein the sign of the roll moment derivative for hovering cranefly is also negative, and the dynamic modal structure includes one weakly unstable oscillatory mode. In other words, the lateral motion of hovering cranefly can be considered as nearly neutral. The recent study [17] has pointed that the wing-wing interaction has the effect on the roll moment derivative, which can turn to be negative compared with the case without the wing-wing interaction and stabilize the hovering hawkmoth in lateral. Considering that the flows around the right and left wing herein are calculated separately, therefore, the value of the negative roll moment derivative
For the previous CFD studies, the dynamic modal structure of cranefly, including one weakly unstable oscillatory mode and two subsidence modes, is different from those of hovering dronefly [8], bumblebee [11] and mosquito [13], while similar to that of hovering honeybee [16]. The reason for the difference has been briefly discussed in the above section, which is the derivative
(1) This paper investigated the lateral dynamics of a model cranefly in hover. The lateral disturbance motion of model cranefly in hover consists of three natural modes, and the flight in lateral is nearly neutral, that is different from most other insects.
(2) The neutral stability is chiefly due to the stability derivative
(3) The stability analysis on model cranefly shows that, during normal-hovering flight, the stability property of lateral disturbance motions is not as robust as that of longitudinal motion, and owing to the opposite sign of
Funding Statement: This work was supported by grants from the National Natural Science Foundation of China (Nos. 11802262 and 11502228).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Taha, H. E., Hajj, M. R., Nayfeh, A. H. (2012). Flight dynamics and control of flapping-wing MAVs: A review. Nonlinear Dynamics, 70(2), 907–939. DOI 10.1007/s11071-012-0529-5. [Google Scholar] [CrossRef]
2. Sun, M. (2014). Insect flight dynamics: Stability and control. Reviews of Modern Physics, 86(2), 615–646. DOI 10.1103/RevModPhys.86.615. [Google Scholar] [CrossRef]
3. Taylor, G. K., Thomas, A. L. R. (2003). Dynamic flight stability in the desert locust Schistocerca gregaria. Journal of Experimental Biology, 206(16), 2803–2829. DOI 10.1242/jeb.00501. [Google Scholar] [CrossRef]
4. Sun, M., Xiong, Y. (2005). Dynamic flight stability of a hovering bumblebee. Journal of Experimental Biology, 208(3), 447–459. DOI 10.1242/jeb.01407. [Google Scholar] [CrossRef]
5. Sun, M., Wang, J. K., Xiong, Y. (2007). Dynamic flight stability of hovering insects. Acta Mechanica Sinica, 23(3), 231–246. DOI 10.1007/s10409-007-0068-3. [Google Scholar] [CrossRef]
6. Faruque, I., Humbert, J. S. (2010). Dipteran insect flight dynamics. Part 1 longitudinal motion about hover. Journal of Theoretical Biology, 264(2), 538–552. DOI 10.1016/j.jtbi.2010.02.018. [Google Scholar] [CrossRef]
7. Faruque, I., Humbert, J. S. (2010). Dipteran insect flight dynamics. Part 2: Lateral-directional motion about hover. Journal of Theoretical Biology, 265(3), 306–313. DOI 10.1016/j.jtbi.2010.05.003. [Google Scholar] [CrossRef]
8. Zhang, Y., Sun, M. (2010). Dynamic flight stability of a hovering model insect: Lateral motion. Acta Mechanica Sinica, 26(2), 175–190. DOI 10.1007/s10409-009-0303-1. [Google Scholar] [CrossRef]
9. Cheng, B., Deng, X. (2011). Translational and rotational damping of flapping flight and its dynamics and stability at hovering. IEEE Transactions on Robotics, 27(5), 849–864. DOI 10.1109/TRO.2011.2156170. [Google Scholar] [CrossRef]
10. Mou, X., Sun, M. (2012). Dynamic flight stability of a model hoverfly in inclined-stroke-plane hovering. Journal of Bionic Engineering, 9(3), 294–303. DOI 10.1242/jeb.054874. [Google Scholar] [CrossRef]
11. Xu, N., Sun, M. (2013). Lateral dynamic flight stability of a model bumblebee in hovering and forward flight. Journal of Theoretical Biology, 319, 102–115. DOI 10.1016/j.jtbi.2012.11.033. [Google Scholar] [CrossRef]
12. Kim, J. K., Han, J. S., Lee, J. S., Han, J. H. (2015). Hovering and forward flight of the hawkmoth Manduca sexta: Trim search and 6-DOF dynamic stability characterization. Bioinspiration & Biomimetics, 10(5), 56012. DOI 10.1088/1748-3190/10/5/056012. [Google Scholar] [CrossRef]
13. Liu, L., Sun, M. (2019). Dynamic flight stability of hovering mosquitoes. Journal of Theoretical Biology, 464, 149–158. DOI 10.1016/j.jtbi.2018.12.038. [Google Scholar] [CrossRef]
14. Bomphrey, R. J., Nakata, T., Phillips, N., Walker, S. M. (2017). Smart wing rotation and trailing-edge vortices enable high frequency mosquito flight. Nature, 544(7648), 92–95. DOI 10.1038/nature21727. [Google Scholar] [CrossRef]
15. Xu, N., Sun, M. (2014). Lateral dynamic flight stability of a model hoverfly in normal and inclined stroke-plane hovering. Bioinspiration & Biomimetics, 9(3), 36019. DOI 10.1088/1748-3182/9/3/036019. [Google Scholar] [CrossRef]
16. Xu, N., Sun, M. (2014). Lateral flight stability of two hovering model insects. Journal of Bionic Engineering, 11(3), 439–448. DOI 10.1016/s1672-6529(14)60056-1. [Google Scholar] [CrossRef]
17. Han, J. S., Han, J. H. (2019). A contralateral wing stabilizes a hovering hawkmoth under a lateral gust. Scientific Reports, 9. DOI 10.1038/s41598-019-53625-0. [Google Scholar] [CrossRef]
18. Kruyt, J. W., van Heijst, G. F.,, Altshuler, D. L., Lentink, D. (2015). Power reduction and the radial limit of stall delay in revolving wings of different aspect ratio. Journal of the Royal Society Interface, 12(105), 20150051. DOI 10.1098/rsif.2015.0051. [Google Scholar] [CrossRef]
19. Han, J. S., Chang, J. W., Cho, H. K. (2015). Vortices behavior depending on the aspect ratio of an insect-like flapping wing in hover. Experiments in Fluids, 56(9), 181. DOI 10.1007/s00348-015-2049-9. [Google Scholar] [CrossRef]
20. Harbig, R. R., Sheridan, J., Thompson, M. C. (2013). Reynolds number and aspect ratio effects on the leading-edge vortex for rotating insect wing planforms. Journal of Fluid Mechanics, 717, 166–192. DOI 10.1017/jfm.2012.565. [Google Scholar] [CrossRef]
21. Ellington, C. P. (1984). The aerodynamics of hovering insect flight. II. Morphological parameters. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 305(1122), 17–40. DOI 10.1098/rstb.1984.0050. [Google Scholar] [CrossRef]
22. Ellington, C. P. (1984). The aerodynamics of hovering insect flight. III. Kinematics. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 305(1122), 41–78. DOI 10.1098/rstb.1984.0051. [Google Scholar] [CrossRef]
23. Sun, M., Tang, J. (2002). Unsteady aerodynamic force generation by a model fruit fly wing in flapping motion. Journal of Experimental Biology, 205(1), 55–70. DOI 10.1242/jeb.205.1.55. [Google Scholar] [CrossRef]
24. Rogers, S. E., Kwak, D., Kiris, C. (1991). Numerical solution of the incompressible Navier–Stokes equations for steady-state and time-dependent problems. 27th Aerospace Sciences Meeting, 89, 463. DOI 10.2514/6.1989-463. [Google Scholar] [CrossRef]
25. Du, G., Sun, M. (2012). Aerodynamic effects of corrugation and deformation in flapping wings of hovering hoverflies. Journal of Theoretical Biology, 300, 19–28. DOI 10.1016/j.jtbi.2012.01.010. [Google Scholar] [CrossRef]
26. Liang, B., Sun, M. (2011). Aerodynamic interactions between contralateral wings and between wings and body of a model insect at hovering and small speed motions. Chinese Journal of Aeronautics, 24(4), 396–409. DOI 10.1016/S1000-9361(11)60047-2. [Google Scholar] [CrossRef]
27. Dudley, R., Ellington, C. (1990). Mechanics of forward flight in bumblebees: I. Kinematics and morphology. Journal of Experimental Biology, 148(1), 19–52. DOI 10.1242/jeb.148.1.19. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |