 Open Access
Open Access
ARTICLE
Mind matters: how anxiety and depression shape low-risk prostate cancer active surveillance adherence in a real-world population
1 Main Line Health System, Wynnewood, PA 19096, USA
2 Philadelphia College of Osteopathic Medicine, Philadelphia, PA 19131, USA
3 Nova Southeastern University Dr. Kiran C. Patel College of Osteopathic Medicine, Fort Lauderdale, FL 33328, USA
4 Lankenau Institute of Medical Research, Penn Wynne, PA 19096, USA
5 Department of Psychiatry, Thomas Jefferson University Hospital, Philadelphia, PA 19107, USA
6 MidLantic Urology, Bryn Mawr, PA 19010, USA
* Corresponding Author: Zachariah Taylor. Email:
Canadian Journal of Urology 2025, 32(1), 21-27. https://doi.org/10.32604/cju.2025.064705
Received 11 December 2024; Accepted 20 January 2025; Issue published 20 March 2025
Abstract
Purpose: While the mental health impact of a prostate cancer diagnosis, including low-risk prostate cancer, is well-documented, the effect of pre-existing anxiety and/or depression on adherence to active surveillance protocols in low-risk prostate cancer patients remains unclear. This study assessed the association between prior anxiety and/or depression and active surveillance adherence in men with low-risk prostate cancer. Methods: We conducted a retrospective, multicenter study involving 426 men diagnosed with low-risk prostate cancer who were recommended active surveillance as the primary management strategy. Active surveillance adherence was defined by completion of both a prostate-specific antigen test and a prostate biopsy within 18 months of diagnosis. Premature treatment was identified as definitive treatment, either through radiation therapy or radical prostatectomy. Results: Men with a prior mental health diagnosis were significantly less likely to adhere to active surveillance than those without such a diagnosis (27.6% vs. 49.5%, p = 0.006). These individuals had lower adherence rates for prostate-specific testing (58.6% vs. 73.4%) and biopsy (27.6% vs. 50.0%) and were more likely to abandon active surveillance in favor of immediate treatment (39.7% vs. 25.0%, p = 0.005). No significant differences were observed between patients with both anxiety and depression versus those with a single diagnosis. Conclusions: Pre-existing anxiety and/or depression is associated with reduced active surveillance adherence and a greater likelihood of premature treatment in men with low-risk prostate cancer. These findings highlight the importance of addressing psychiatric factors in low-risk prostate cancer management and suggest avenues for future research.Keywords
Prostate cancer (PCa) is the most common cancer among men in the United States, with an estimated 299,010 new cases in 2024.1 Given PCa’s often indolent nature, recommended treatments vary according to the patient’s risk profile. Low-risk prostate cancer (LRPCa) is characterized by a prostate-specific antigen (PSA) < 10 ng/mL, grade group 1, and clinical stage T1-T2a.2 The American Urological Association and the National Comprehensive Cancer Network (NCCN) recommend active surveillance (AS) for managing LRPCa. Multiple studies have demonstrated that AS is a safe option for appropriately selected men, preserving the option for curative treatment if needed.2 AS protocols can vary, but generally include regular PSA tests and repeat prostate biopsies within approximately one year.3 For instance, the Prostate Cancer Research International Active Surveillance (PRIAS) study recommends PSA testing every three months for the first two years, digital rectal exams every six months, and a repeat biopsy one year after diagnosis.4
The adverse impact of a cancer diagnosis on mental health is well-documented.5 For men undergoing treatment for PCa, side effects such as urinary incontinence and sexual dysfunction can lead to increased anxiety and depression.6–8 While AS for LRPCa avoids the immediate side effects associated with whole gland therapy, it has nonetheless been linked to elevated anxiety in patients.9 Although a diagnosis and potential treatment for localized PCa is associated with mental health decline, the effect of pre-existing anxiety and/or depression on the treatment course in men with LRPCa is less clear.
This study aims to assess whether men with a prior diagnosis of anxiety and/or depression experience different treatment courses during AS for LRPCa compared to those without documented mental health conditions.
We conducted a retrospective cohort study of men diagnosed with localized LRPCa at our institution between October 2018 and June 2023. Patients’ demographic and health information, including mental health diagnoses, were recorded prior to their PCa diagnosis. Men with documented diagnoses of anxiety and/or depression were included. Those with other mental health conditions (e.g., bipolar disorder, substance use disorder, or schizophrenia) or a genetic predisposition to PCa (e.g., BRCA mutations or high-risk genomic testing results) were excluded. We recorded each patient’s PSA value at diagnosis, as well as subsequent PSA values, repeat biopsy results, and the timing of these events. Treatment details, including radical prostatectomy or radiation therapy, were also documented. Patients who received focal or investigational therapies were excluded.
LRPCa was defined per the American Urologic Association as having all of the following: clinical T stage T2a or lower, PSA < 10 ng/mL, and no grade group higher than 1 in any core.
AS adherence was defined as completing both a PSA test and a prostate biopsy within 18 months of diagnosis. Patients missing either component were classified as nonadherent, and adherence to PSA and biopsy protocols was recorded separately.
Premature treatment was defined as whole gland treatment (radiation therapy or radical prostatectomy) in men diagnosed with LRPCa. Patients who transitioned to intermediate or higher risk during AS and then received treatment were classified as AS adherent.
Descriptive statistics were used to characterize men with and without a diagnosis of anxiety and/or depression. Baseline and adherence differences between the two groups were assessed using two-sample t-tests for continuous variables and chi-square tests for categorical variables. Sub-group analyses for no mental health diagnosis, anxiety only, depression only, and both anxiety and depression were conducted similarly. A multinomial logistic regression model was developed to explore associations between nonadherence, adherence, and premature treatment, adjusting for potential confounders. A univariable model was first run, followed by a multivariable model including age, race, family history of PCa, and COPD, selected based on clinical significance in prior studies or significant baseline differences. Analyses were conducted in Stata 18.0 (StataCorp, College Station, TX, USA), with p-values < 0.05 considered statistically significant. Regression results are presented with 95% confidence intervals.
This study received institutional review board approval (IRB #: E-23-5351).
A total of 426 men with LRPCa were included in the study, of whom 58 had a diagnosis of anxiety and/or depression. Demographic and patient characteristics are presented in Table 1. The average age was 68.3 years in the cohort without anxiety and/or depression and 67.3 years in those with anxiety and/or depression (p = 0.311). Racial distribution was similar between groups, with the majority being white males (82.8% in the no anxiety and/or depression group and 87.9% in the anxiety and/or depression group; p = 0.399). There were no significant differences in PSA levels at diagnosis (p = 0.334) or in family history of prostate cancer (p = 0.375). Men with anxiety and/or depression had significantly higher rates of COPD (8.6% vs. 2.2%, p = 0.008), while no differences were observed in other comorbidities, including hypertension (57.9% vs. 65.5%, p = 0.275), type II diabetes (14.4% vs. 18.9%, p = 0.366), chronic kidney disease (3.5% vs. 1.7%, p = 0.473), or coronary artery disease (9.0% vs. 8.8%, p = 0.957).
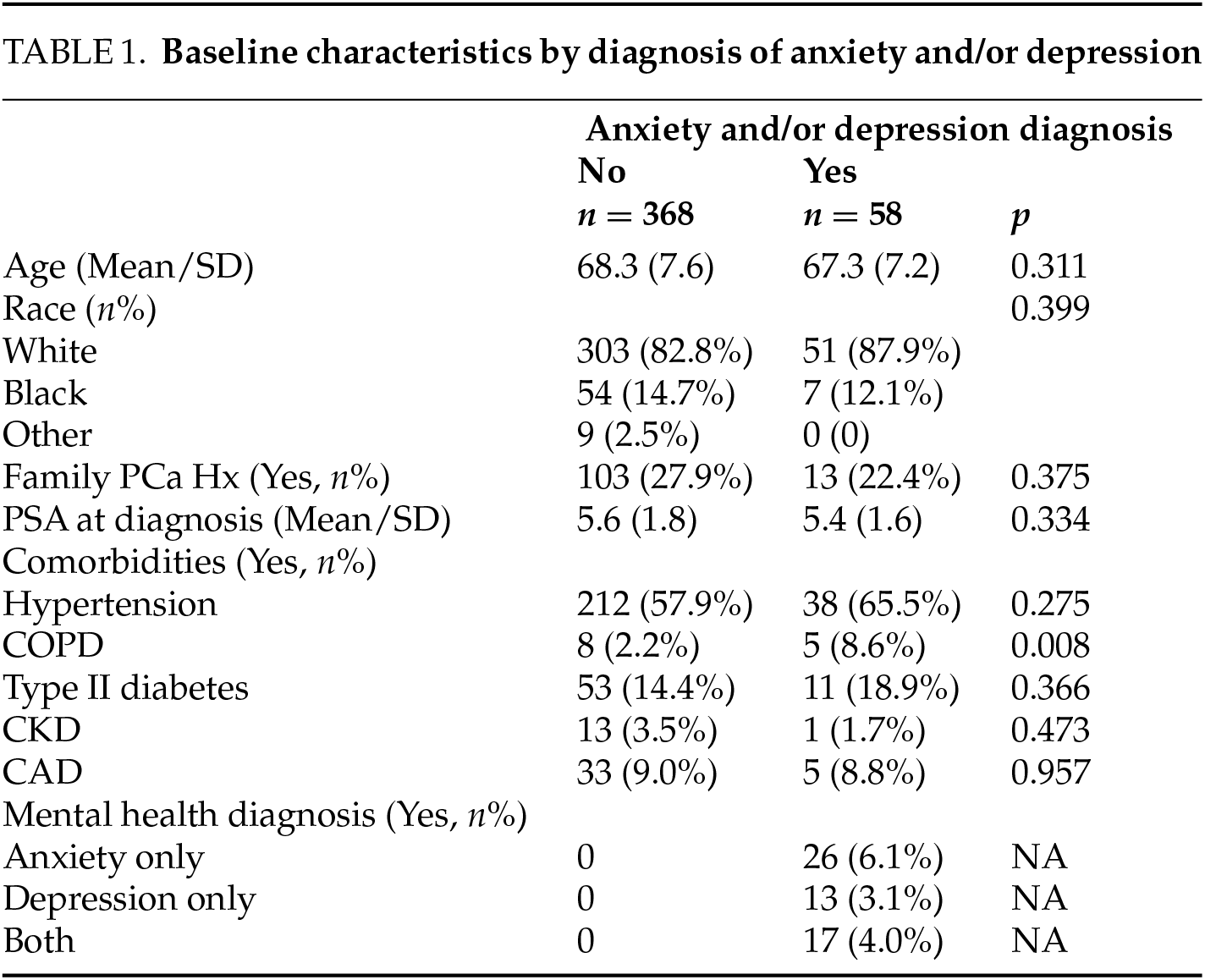
Men with anxiety and/or depression were significantly less likely to adhere to AS protocols compared to those without these diagnoses. Only 27.6% of those with anxiety and/or depression were fully adherent to their AS protocol, a rate significantly lower than the 49.5% adherence observed in men without these mental health conditions (p = 0.006). Additionally, men with anxiety and/or depression were more likely to forgo AS and opt for whole gland treatment (39.7% vs. 25.0%, p = 0.005). PSA adherence was achieved by 58.6% of men with anxiety and/or depression, compared to 73.4% of those without such diagnoses (p = 0.063). Among those who chose premature treatment, 34.8% of men with anxiety and/or depression underwent radical prostatectomy, while 65.2% received radiation therapy. See Table 2.
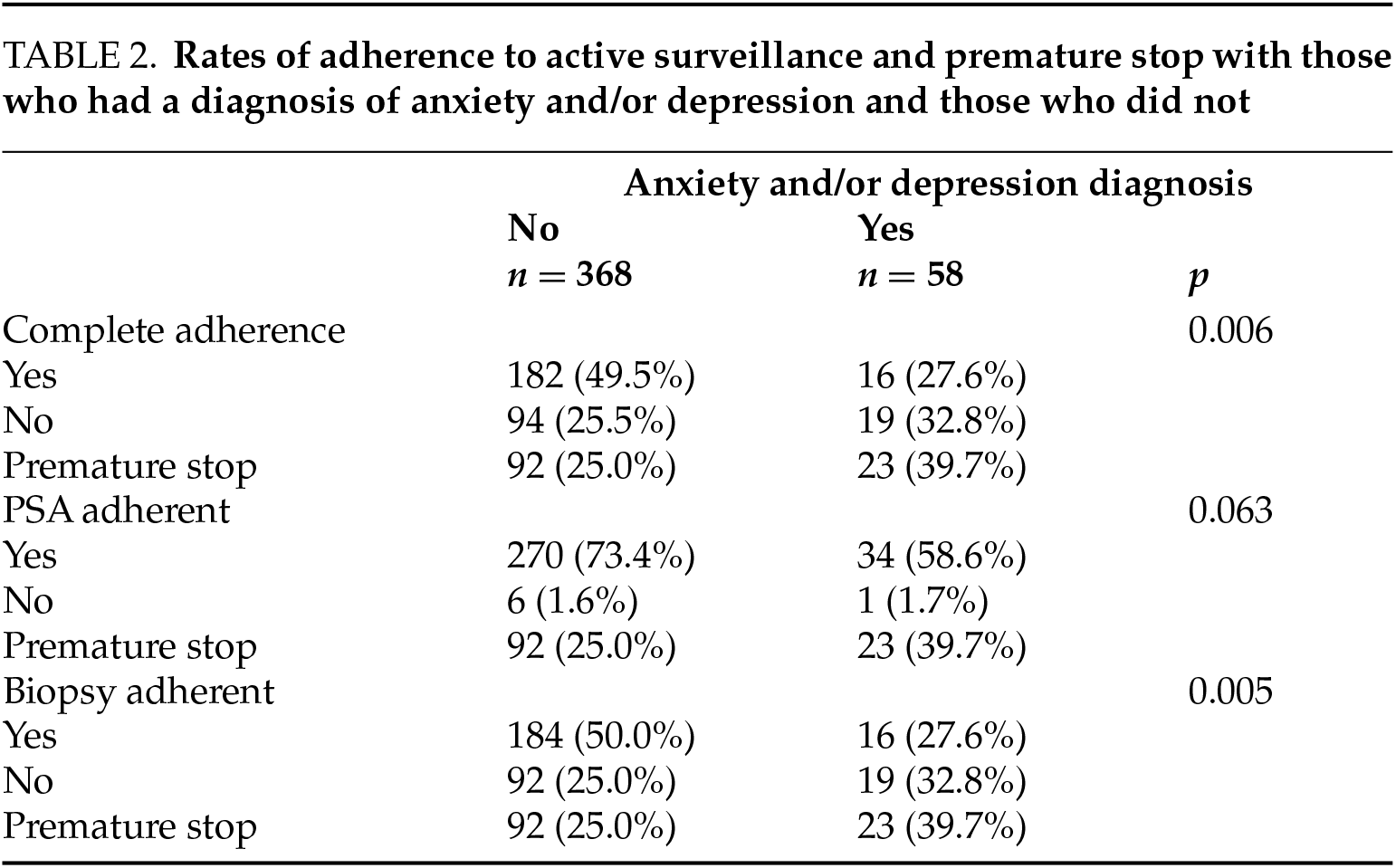
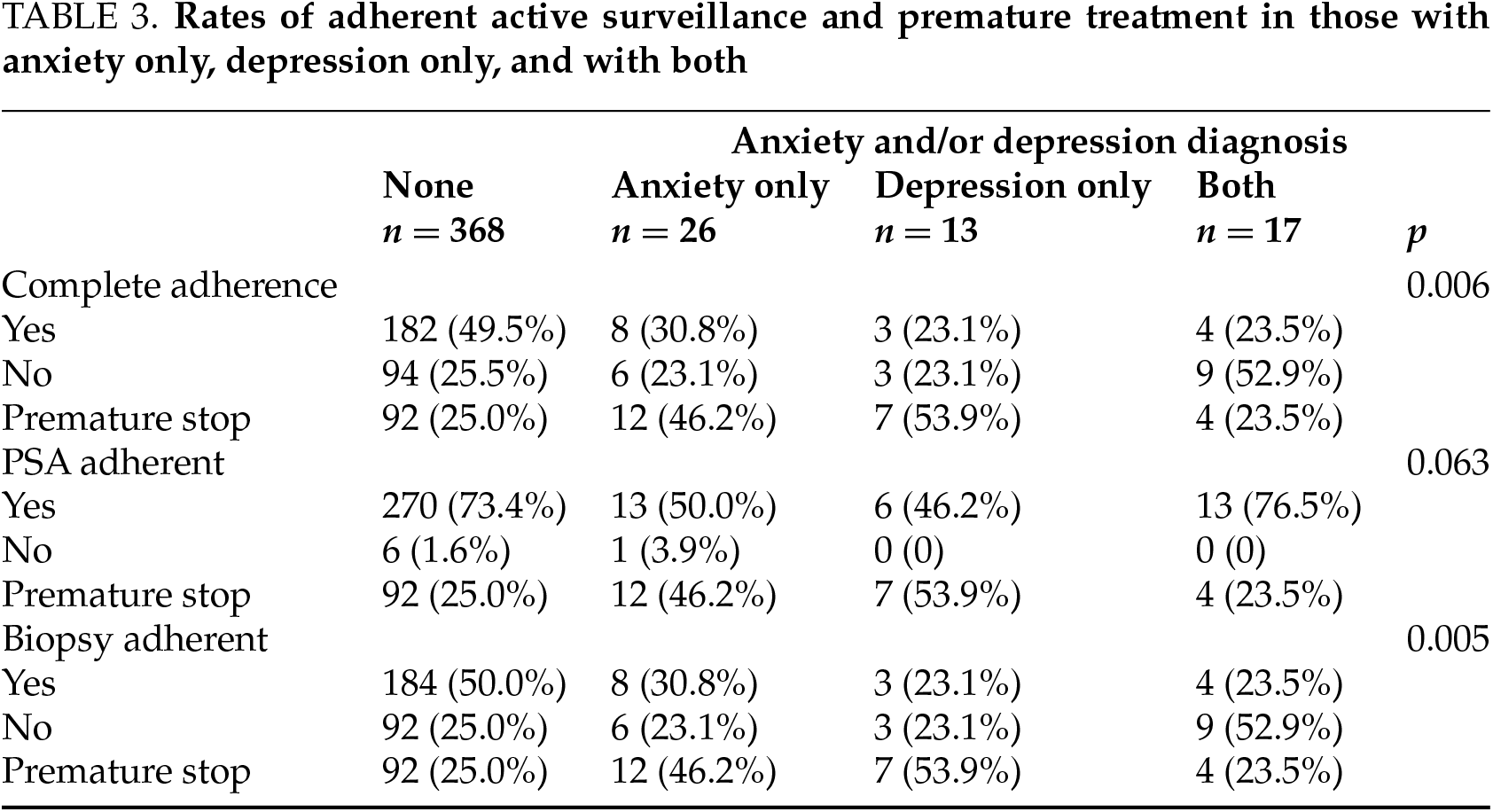
Further evaluation showed that complete AS adherence was achieved in 30.8% of patients with anxiety only, compared to 23.1% of those with depression only. Patients diagnosed with both anxiety and depression had an adherence rate of 23.5%. PSA adherence was observed in 50.0% of patients with anxiety only, versus 46.2% of those with depression only. A premature stop to AS occurred in 46.2% of anxiety-only patients and 53.9% of those with depression only. See Table 3.
Multivariable regression analysis indicated that men with anxiety and/or depression had a relative risk ratio of 2.5 for nonadherence to AS (95% CI: 1.2–5.2, p < 0.05), as shown in Figure 1. Age, race/ethnicity, family history, and COPD were not significantly associated with nonadherence in the multivariable model. The analysis also revealed a relative risk ratio of 3.0 (95% CI: 1.5–6.1, p < 0.05) for premature AS discontinuation among those with anxiety and/or depression, as shown in Figure 2. Additionally, Black race was associated with a relative risk ratio of 2.5 (95% CI: 1.3–4.8, p < 0.05) for premature AS discontinuation compared to white individuals. Family history and COPD were not significantly associated with higher rates of premature AS discontinuation.

FIGURE 1. Forrest plot demonstrating relationship between nonadherence and adherence to active surveillance protocols from multinomial logistic regression analysis. Point demonstrates estimated relative risk with associated 95% confidence interval

FIGURE 2. Forrest plot demonstrating relationship between premature stop versus adherence to active surveillance protocols from multinomial logistic regression. Point demonstrates estimated relative risk with associated 95% confidence interval
To our knowledge, this is the first study to demonstrate an association between a pre-existing diagnosis of anxiety and/or depression and the treatment course of men with LRPCa. We found that men with a prior diagnosis of anxiety and/or depression exhibited higher rates of nonadherence to AS protocols and more frequently received premature treatment with either radiation therapy or radical prostatectomy.
Anxiety and depression are common diagnoses in the United States, affecting an estimated 6.8 million individuals with generalized anxiety disorder (GAD) and 21 million with major depressive disorder (MDD) annually, according to the Anxiety and Depression Association of America and the National Institute of Mental Health. Prostate cancer is most frequently diagnosed between the ages of 65 and 74, with previous studies reporting anxiety rates of 2.69% and depression rates of 4.4% in men within this age range.10,11 In our cohort, 6.1% had anxiety only, 3.1% had depression only, and 4.0% had both anxiety and depression. Despite the prevalence of these conditions, there is a lack of literature on their impact on AS adherence in men with LRPCa. Studies have shown that anxiety during AS is linked to a quicker transition to active treatment. For example, Latini et al. found that increasing anxiety scores independently predicted treatment initiation, but the study did not examine the impact of pre-existing anxiety or depression before LRPCa diagnosis.12
The mental health burden associated with AS is well documented,12 and some reports suggest that men on AS have greater unmet needs than those with more advanced cancer.13 Studies have shown that anxiety may increase in men with PCa due to uncertainty, loss of control, and insufficient support and education regarding initial treatment planning.12 Mental health disorder prevalence ten years post-diagnosis is highest among patients undergoing watchful waiting, compared to those who had radical prostatectomy or radiotherapy.14 AS protocols are variable and can differ among providers;15 however, all generally include regular PSA testing and repeat biopsies.16 Each test may provoke patient anxiety due to the uncertainty it brings and the potential for disease progression.17
When analyzed separately, patients with anxiety only or depression only demonstrated significantly lower AS adherence and higher rates of premature AS cessation compared to those without anxiety or depression. Notably, having both anxiety and depression did not lead to significantly different adherence rates compared to those with either condition alone.
Our findings suggest an opportunity to better support men with anxiety and/or depression who are undergoing AS for LRPCa. Diagnoses of anxiety and/or depression may contribute to overtreatment via radical prostatectomy or radiation therapy or may increase the risk of AS nonadherence. Adherence to AS protocols might be improved through psychosocial interventions, particularly for patients with pre-existing mental health conditions.
The need for psychiatric support in cancer care is well recognized. A UK study found that 73% of patients with both depression and cancer were not receiving adequate treatment for their depression.18 While the integration of psychiatry and oncology is not new—the American Psychosocial Oncology Society was established in the 1980s to coordinate care across disciplines19—the use of integrative care models remains limited in many urology practices, particularly regarding psychiatric services.18,20 An integrated care model involves a coordinated network of healthcare providers delivering comprehensive services to specific patient populations.21 Tumor boards are an example of an integrative model used to treat urologic cancers, showing clinical impact on both diagnosis and treatment decisions compared to those made by individual urologists.22 The documented links between psychiatric illness and genitourinary cancers, such as the neuropsychiatric side effects of androgen deprivation therapy in PCa and higher suicide rates in bladder cancer patients, underscore the need for enhanced mental healthcare in urology.23,24 Furthermore, studies suggest that utilizing psychiatric care in emergency or inpatient settings may correlate with higher prostate cancer-specific mortality, highlighting the importance of accessible mental healthcare.25 Integrating mental health services into urology, whether through on-site psychiatry consultations or proactive mental health screening by non-psychiatrists, could improve outcomes for PCa patients. Based on our findings, we suggest that urologist should recommend psychiatric assistance for men with a new diagnosis of LRPCa.
Current guidelines recommend PSA testing and repeat prostate biopsies to monitor disease progression, although no standardized protocol for the timing of these tests exists, with intervals varying across major AS cohorts.26–29 We did not consider multiparametric MRI (mpMRI) as a substitute for repeat PSA and biopsy due to its unclear role in AS for LRPCa.30 Current guidelines on AS for LRPCa recommend using mpMRI to supplement risk stratification but do not suggest it should replace periodic biopsies.2 Ideally, AS protocols should be personalized, and further studies are needed to define the role of mpMRI in this setting.
Despite the low AS adherence rates in men with a diagnosis of anxiety and/or depression, these findings do not support offering whole gland treatment in leu of AS. Rather, these findings suggest that these men may need a more comprehensive approach with involvement of other health professionals with psychiatric training. It should be noted that even in the men without a diagnosis of anxiety and/or depression, adherence rates were low. Less than half of these men had complete adherence. While outside of the scope of this article, this finding does suggest that continued work should be done to improve AS adherence in all men.
There are several limitations to this study. First, all patients were from a specific geographic region in southeastern Pennsylvania, which may limit generalizability. Additionally, diagnoses of anxiety and depression were not verified using DSM-5 criteria for GAD and MDD, nor were patients assessed with standardized tools such as the Patient Health Questionnaire-9 (PHQ-9) or Generalized Anxiety Disorder-7 (GAD-7). Therefore, any documented history of anxiety and/or depression was treated uniformly. Future prospective studies should incorporate objective mental health assessments like the PHQ-9 or GAD-7 for patients eligible for AS. Furthermore, we did not account for individuals who developed anxiety and/or depression during the study period, nor did we evaluate the impact of pharmacotherapy on anxiety and/or depression. Finally, as a retrospective study, there are inherent limitations and potential biases associated with the study design.
This study highlights a gap in care, as men with a prior diagnosis of anxiety and/or depression show lower AS adherence rates and higher rates of premature treatment initiation. Integrating mental health support into structured AS guidelines to address the unique psychiatric needs of these patients may improve AS adherence and optimize outcomes in LRPCa management.
Acknowledgement
Not applicable.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: Zachariah Taylor, Laurence Belkoff, Kayla Meyer, Ryan Wong, Natalina Contoreggi, Sharon Larson, and Ilia Zeltser; data collection: Zachariah Taylor, Kayla Meyer, Danielle Terrenzio, Ryan Wong; analysis and interpretation of results: Zachariah Taylor, Stephanie Kjelstrom, Laurence Belkoff, Ilia Zeltser; draft manuscript preparation: Zachariah Taylor, Stephanie Kjelstrom, Natalina Contoreggi and Ilia Zeltser. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The data set generated and analyzed for this study can be made available from the corresponding author on reasonable request.
Ethics Approval
This study was found to be exempt by the Main Line Health Institutional Review Board #E-23-5351.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. SEER Cancer Stat Facts: Prostate Cancer. National Cancer Institute [Internet]. Bethesda, MD [cited 2024 Sep 19]. Available from: https://seer.cancer.gov/statfacts/html/prost.html. [Google Scholar]
2. Eastham JA, Auffenberg GB, Barocas DA et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol 2022;208(1):10–18. [Google Scholar]
3. Walker CH, Marchetti KA, Singhal U, Morgan TM. Active surveillance for prostate cancer: selection criteria, guidelines, and outcomes. World J Urol 2022;40(1):35–42. [Google Scholar]
4. van den Bergh RC, Roemeling S, Roobol MJ, Roobol W, Schröder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol 2007;52(6):1560–1563. [Google Scholar]
5. Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (Review). Oncol Lett 2015;9(4):1509–1514. [Google Scholar]
6. Hanly N, Mireskandari S, Juraskova I. The struggle towards ‘the New Normal’: a qualitative insight into psychosexual adjustment to prostate cancer. BMC Urol 2014;14(1):56. [Google Scholar]
7. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol 2008;4(4):561–568. [Google Scholar]
8. Zhang AY, Ganocy S, Fu AZ et al. Mood outcomes of a behavioral treatment for urinary incontinence in prostate cancer survivors. Support Care Cancer 2019;27(12):4461–4467. [Google Scholar]
9. Tan HJ, Marks LS, Hoyt MA et al. The relationship between intolerance of uncertainty and anxiety in men on active surveillance for prostate cancer. J Urol 2016;195(6):1724–1730. [Google Scholar]
10. Grenier S, Payette MC, Gunther B et al. Association of age and gender with anxiety disorders in older adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2019;34(3):397–407. [Google Scholar]
11. Li S, Zhang X, Cai Y, Zheng L, Pang H, Lou L. Sex difference in incidence of major depressive disorder: an analysis from the Global Burden of Disease Study 2019. Ann Gen Psychiatry 2023;22(1):53. [Google Scholar]
12. Latini DM, Hart SL, Knight SJ et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol 2007;178(3 Pt 1):826–831; discussion 831–822. [Google Scholar]
13. Pickles T, Ruether JD, Weir L, Carlson L, Jakulj F. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU Int 2007;100(3):544–551. [Google Scholar]
14. Ravi P, Karakiewicz PI, Roghmann F et al. Mental health outcomes in elderly men with prostate cancer1Equal contribution. Urol Oncol: Semin Original Investig 2014;32(8):1333–1340. [Google Scholar]
15. Cheah WL, Ling NC, Chang KH. The supportive care needs for prostate cancer patients in Sarawak. Chin Clin Oncol 2016;5(1):7. [Google Scholar]
16. Luckenbaugh AN, Auffenberg GB, Hawken SR et al. Variation in guideline concordant active surveillance followup in diverse urology practices. J Urol 2017;197(3 Pt 1):621–626. [Google Scholar]
17. Morash C, Tey R, Agbassi C et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J 2015;9(5–6):171–178. [Google Scholar]
18. Walker J, Hansen CH, Martin P et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014;1(5):343–350. [Google Scholar]
19. APOS: American Psycho-Oncology Society: Mission & Vision [Internet]. [cited 2024 Oct 8]. Available from: https://apos-society.org/mission-vision/. [Google Scholar]
20. Fernando A. Mental health and cancer: why it is time to innovate and integrate—a call to action. Eur Urol Focus 2020;6(6):1165–1167. [Google Scholar]
21. Enthoven AC. Integrated delivery systems: the cure for fragmentation. Am J Manag Care 2009;15(10 Suppl):S284–290. [Google Scholar]
22. Kurpad R, Kim W, Rathmell WK et al. A multidisciplinary approach to the management of urologic malignancies: does it influence diagnostic and treatment decisions? Urol Oncol 2011;29(4):378–382. [Google Scholar]
23. Dinh KT, Reznor G, Muralidhar V et al. Association of androgen deprivation therapy with depression in localized prostate cancer. J Clin Oncol 2016;34(16):1905–1912. [Google Scholar]
24. Ramakrishnan VM, Trinh QD. Suicide risk among patients with genitourinary malignancies: where do we stand? Eur Urol Focus 2020;6(6):1145–1146. [Google Scholar]
25. Klaassen Z, Wallis CJD, Goldberg H et al. The impact of psychiatric utilisation prior to cancer diagnosis on survival of solid organ malignancies. Br J Cancer 2019;120(8):840–847. [Google Scholar]
26. Bul M, Zhu X, Valdagni R et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63(4):597–603. [Google Scholar]
27. Klotz L, Vesprini D, Sethukavalan P et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33(3):272–277. [Google Scholar]
28. Newcomb LF, Thompson IM Jr., Boyer HD et al. Outcomes of active surveillance for clinically localized prostate cancer in the prospective, multi-institutional canary PASS cohort. J Urol 2016;195(2):313–320. [Google Scholar]
29. Welty CJ, Cowan JE, Nguyen H et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015;193(3):807–811. [Google Scholar]
30. Inoue LYT, Lin DW, Newcomb LF et al. Comparative analysis of biopsy upgrading in four prostate cancer active surveillance cohorts. Ann Intern Med 2018;168(1):1–9. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools