 Open Access
Open Access
ARTICLE
Transperineal prostate biopsy without routine antibiotics demonstrates decreased infection risk
1Section of Urologic Oncology, Rutgers Cancer Institute of New Jersey and Rutgers Robert Wood Johnson University Hospital, New Brunswick, NJ 08901, USA
2 Division of Urology, Department of Surgery, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ 08901, USA
3 Division of Dow Health Services Research, Department of Urology, University of Michigan, Ann Arbor, MI 48104, USA
* Corresponding Author: Benjamin J. Lichtbroun. Email:
Canadian Journal of Urology 2025, 32(1), 55-62. https://doi.org/10.32604/cju.2025.064701
Received 02 October 2024; Accepted 30 November 2024; Issue published 20 March 2025
Abstract
Introduction: Infections are the most feared complication of transrectal prostate biopsies, along with growing concerns of antibiotic resistance. Our institution transitioned to a transperineal approach without use of perioperative antibiotics or bowel preparations. We aimed to compare the safety outcomes associated with transperineal and transrectal prostate biopsy techniques. Materials and Methods: A retrospective analysis of patients who underwent transrectal and transperineal prostate biopsies at our institution from 2019–2022 was performed. Results: We identified 319 patients—174 transrectal and 145 transperineal. 8 patients who had transperineal biopsy (5.5%) received peri-operative antibiotics, compared to 100% with transrectal biopsy. 35.86% of transperineal patients received a bowel preparation, compared to 100% in the transrectal group. 44.14% and 49.43% of patients received a prior prostate biopsy in the transperineal and transrectal groups, respectively. Patients in the transperineal biopsy group had zero infectious complications, 1 ER visit, and zero 30-day readmissions. This is compared to 9 infectious complications (5.17%, p = 0.005), 8 ER visits (4.60%, p = 0.036), and 7 30-day readmissions (4.02%, p = 0.015) in the transrectal group. Conclusions: In a single institution series, patients undergoing transperineal biopsy had fewer infectious complications compared to those undergoing transrectal biopsy. Despite only a small percentage of patients receiving perioperative antibiotics and a majority of patients not receiving a bowel preparation in the transperineal group, there were zero infectious complications or 30-day readmissions. With greater infectious complications with transrectal biopsy and growing antibiotic resistance, we underline the safety of transperineal prostate biopsy which can largely be done without perioperative antibiotics or a bowel preparation.Keywords
Prostate biopsies are a mainstay in the detection of prostate cancer.1 Historically, urologists performed most prostate biopsies via the transrectal approach, necessitating passage of the biopsy needle through the rectal mucosa. Consequently, there is risk of passage of fecal material and gastrointestinal bacteria into the prostate and urinary tract, which can lead to infectious complications, such as post-prostate biopsy sepsis, urinary tract infections (UTIs), and prostatitis.2 The reported rate of post-prostate biopsy sepsis after a transrectal biopsy in multiple large series is 2%–5%.3 Rectal bleeding is also a concern after transrectal prostate biopsies with a reported 1.3%–45% incidence and 2.5% of patients citing this as a major or moderate problem.4 In recent years, much of the urologic community has argued for a return to the transperineal approach to prostate biopsies in order to avoid the necessary passage of the biopsy needle through rectal mucosa, thus theoretically decreasing the risk of infectious complications.
With growing fluoroquinolone resistance and the costly nature of post-biopsy sepsis, infectious complications after biopsy are a major public health concern.5 The AUA currently has no clear recommendation between the two modalities.1 In Europe, transperineal biopsies are preferred over the transrectal route.6 Recently, the results of the ProBE-PC and PREVENT trials came out, which were the first randomized controlled trials directly comparing transrectal vs. transperineal prostate biopsies.7,8 They found no cases of sepsis in either cohort. They also found no significant difference in infectious complications, prompting many to call into question the benefit of the transperineal biopsy.8,9 With zero cases of sepsis in either cohort, the results of these studies deviate significantly from previously reported rates of sepsis with a transrectal biopsy.3 The PREVENT trial also utilized a study population that excluded patients with a prior biopsy and those who had acute bacterial prostatitis within the past 6 months. Despite excluding some of the higher risk patients from the study, rates of infectious complications were 1.4% vs. 0% in favor of the transrectal approach with a p value nearing statistical significance at 0.059.
Our institution moved from a transrectal approach to a transperineal approach in which the transperineal group did not receive a routine bowel preparation or peri-operative antibiotics. We retrospectively reviewed rates of infectious complications between these two real-world cohorts, which included all patients who received a prostate biopsy. We hypothesize that transperineal prostate biopsies without routine administration of antibiotics or a bowel preparation will have a superior safety profile with regard to infectious complications than transrectal prostate biopsies with higher use of antibiotics.
A retrospective analysis was performed of patients who underwent a transrectal prostate biopsy from January 2019–December 2021 or a transperineal prostate biopsy from January 2022–September 2022 at a single academic institution. All patients who underwent either a transrectal or transperineal biopsy during the specified periods were included in the analysis irrespective of past medical history, with the only exclusion criteria being a surgically absent rectum.
Methodology of transrectal and transperineal biopsies
All biopsies were performed in an ambulatory surgical center. The same group of surgeons performed both transrectal and transperineal biopsies, as the institution transitioned to the adoption of transperineal biopsies during the specified time period.
All patients received a local anesthetic injection. Our care pathway recommended conscious sedation, but the decision was ultimately left to the anesthesia team who administered general anesthesia where they deemed it appropriate.
Patients in the transrectal group all received intravenous peri-procedural antibiotics with an intravenous aminoglycoside unless there was a contraindication to this class of medications or there was a prior urine culture that indicated resistance. No patient in either cohort received a pre-operative rectal swab. Patients in the transperineal group did not routinely receive peri-procedural antibiotics, but this was ultimately left up to the discretion of the performing urologist. The choice of aminoglycoside was also at the discretion of the performing urologist but was guided by the institution-specific antibiogram and susceptibility patterns.
Patients in the transrectal group were all given instructions to perform a bowel preparation before the biopsy, which consisted of an enema per rectum. Patients in the transperineal group did not routinely receive a bowel preparation, but this was ultimately left up to the discretion of the performing urologist.
Nearly all biopsies were performed using MRI-guided fusion technology, and all patients who underwent a biopsy were included irrespective of MRI findings. Transrectal and transperineal biopsies were performed using standardized 12- or 14-core biopsy templates, with additional targeted biopsies taken at the discretion of the performing urologist.
Ethical approval was obtained from the local Institutional Review Board (IRB No. Pro2022000891).
Data was retrieved from the electronic medical record. Our primary outcome was infectious complications (including both sepsis and probable UTI) within 30 days. Secondary outcomes were ED visits within 30 days, hospital admissions within 30 days and adverse events within 30 days. Antibiotic use was recorded. Demographic information and information about urologic history were collected. Data about use of MRI, procedure time, bowel preparation use, and procedure-related information was collected.
Infectious complications were defined as the presence of either a UTI or sepsis. A presumed diagnosis of sepsis or UTI was made if based upon the assessment of the provider at the time of presentation, this was the most likely diagnosis and therefore was documented as such. Asymptomatic bacteriuria alone was not considered an infection.
Procedure time was defined as when the patient entered into the operating room until the patient was taken out of the operating room including induction, positioning, software registration, and the biopsy.
Patient demographics and clinicopathologic characteristics were presented as a median and interquartile range for continuous variables or as frequency and percent of the total group for categorical variables. Univariate analyses included Chi-square tests for categorical variables, and Mann-Whitney U tests for continuous variables, with a p < 0.05 considered statistically significant. Fisher’s exact test was used where the event frequency was less than 5. Because the transperineal and transrectal biopsy templates are different, we did not perform hypothesis testing between the location of positive cores between the two groups. Analyses were performed using DATAtab (DATAtab team, Graz, Austria).
The final cohort included 319 patients, of which 145 (45.5%) underwent a transperineal biopsy and 174 (54.5%) underwent a transrectal biopsy. Demographic and clinicopathologic information for each cohort is described in Table 1. In both groups, patients had similar age and BMI. 100% of patients received peri-procedural antibiotics in the transrectal group compared to 5.88% in the transperineal group (p < 0.001). 35.86% of patients received a bowel preparation in the transperineal group compared to 100% in the transrectal group (p < 0.001). 44.14% and 49.43% of patients received a prior prostate biopsy in the transperineal and transrectal groups, respectively. Overall, 100% and 82% of patients received an MRI-guided biopsy in the transperineal and transrectal groups, respectively. There was a higher proportion of patients in the transrectal group that had a PI-RADS lesion ≥ 3 on pre-operative MRI—93.01% vs. 75.17% (p < 0.001). Mean procedure time was longer in the transrectal group than the transperineal group—31 min vs. 24 min (p < 0.001). There were similar rates of clinically significant prostate cancer detected in both groups.
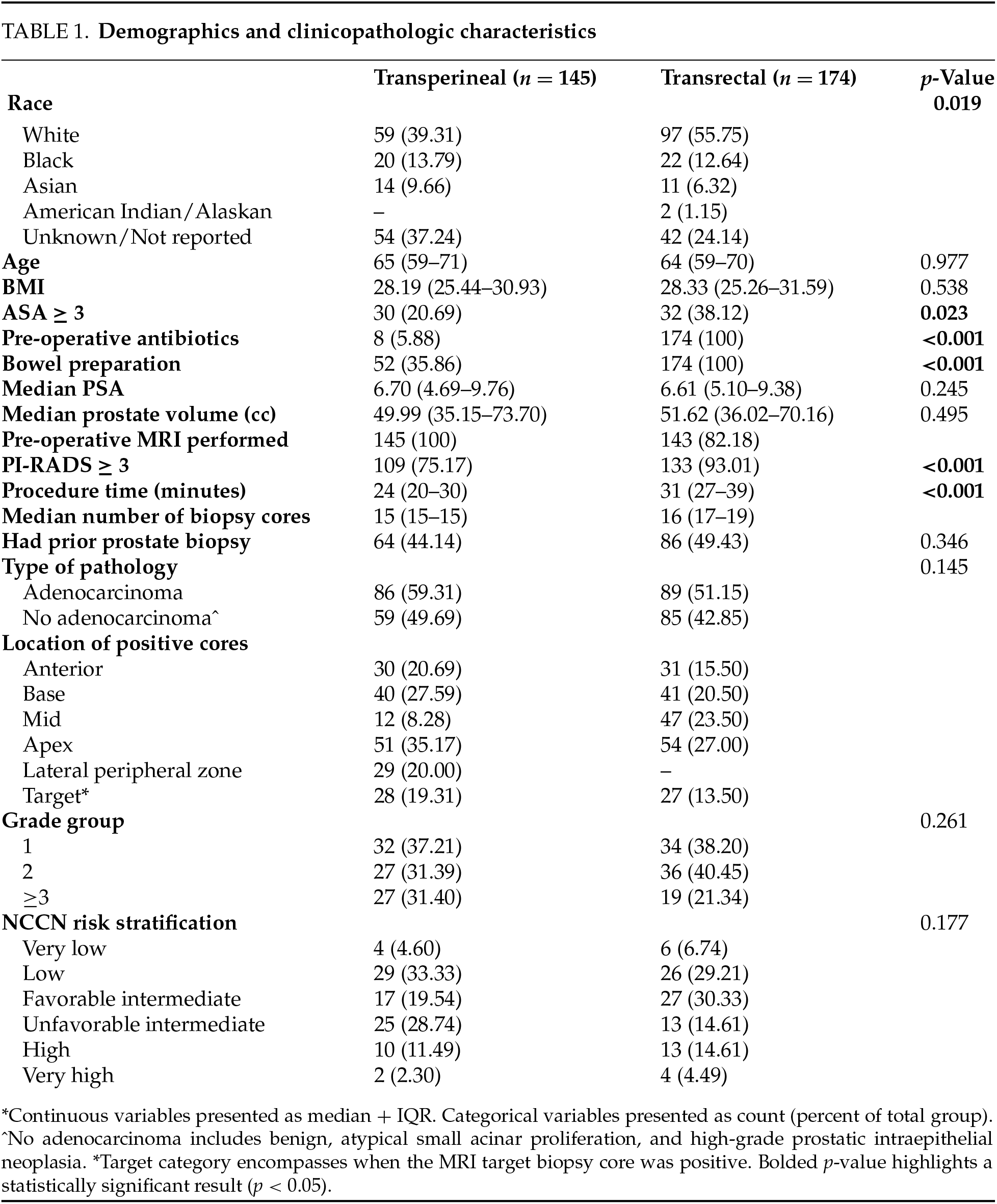
There were less post-operative adverse events in the transperineal biopsy group compared to the transrectal biopsy group—17 (12.41%) vs. 75 (43.10%) (p = 0.010) (Figure 1). There were 0 infectious complications in the transperineal biopsy group and 9 (5.17%) infectious complications in the transrectal biopsy group (p = 0.005) (Figure 1). Specifically, there were 4 (2.30%) episodes of sepsis in the transrectal group compared to 0 in the transperineal group (p = 0.129) and 5 (2.87%) UTIs in the transrectal group compared to 0 in the transperineal group (p = 0.066).
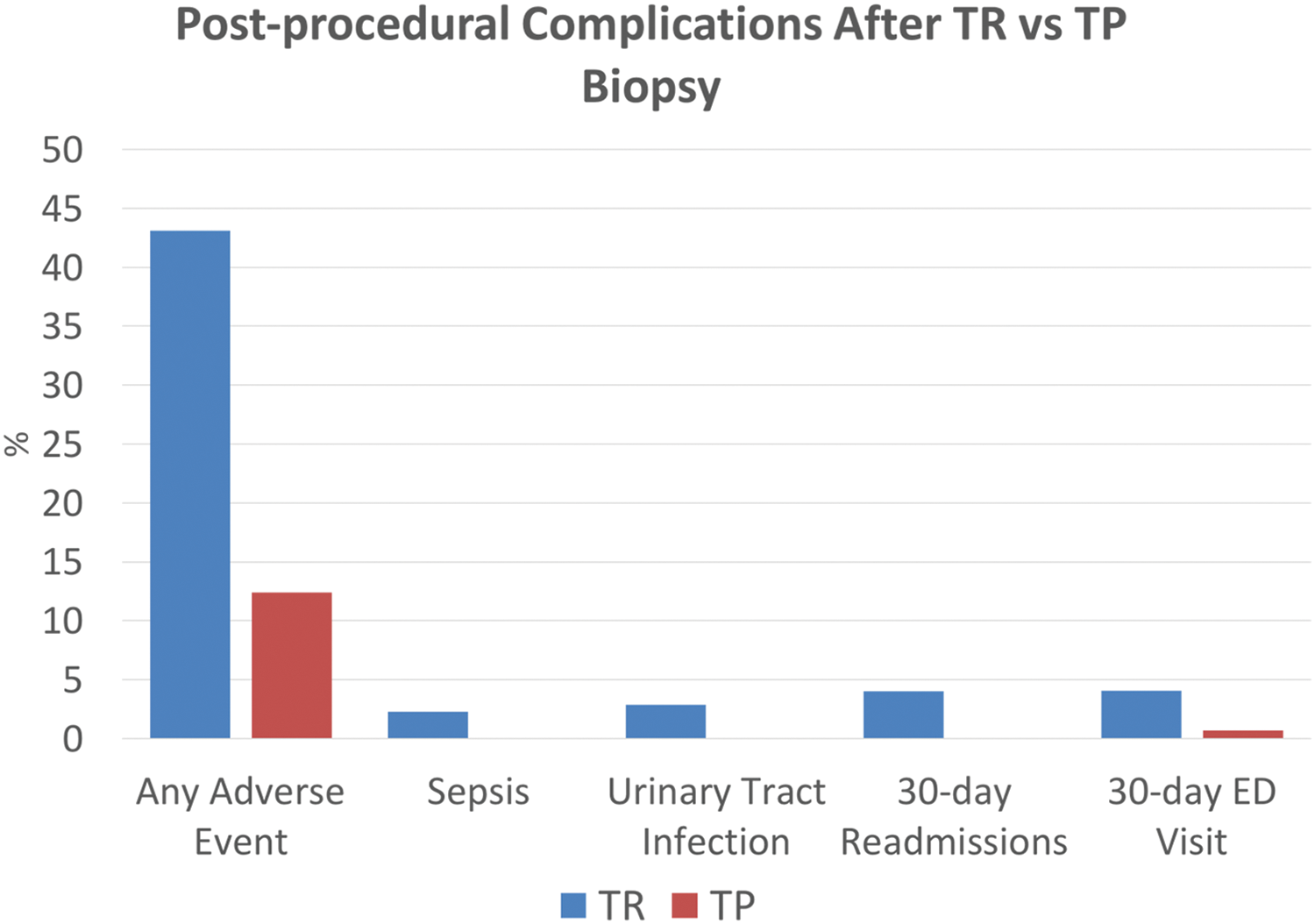
FIGURE 1. Percentage of patients who had a post-procedural complication between transperineal and transrectal prostate biopsies
There was 1 ER visit in the transperineal group (0.69%) and 8 in the transrectal group (4.60%) (p = 0.043) (Figure 1). ER visits for transrectal patients were primarily due to infectious concerns, including one for dysuria and fever, two visits for UTI, and five for sepsis. There were no 30-day readmissions in the transperineal biopsy group and 7 (4.02%) in the transrectal biopsy group (p = 0.017) (Figure 1). Among the transrectal group, patients who presented to the ER were often admitted, with one hospitalization for management of fever and dysuria, one for UTI, and five for sepsis.
In this study, we retrospectively assessed rates of infectious complications from transrectal and transperineal biopsies at our institution. In the transrectal group, 100% of patients received at least peri-procedural antibiotic prophylaxis, compared to 5.5% in the transperineal group. Despite this, and despite eliminating a bowel preparation as part of our routine prostate biopsy pathway with the transperineal approach, we still found significant differences with regard to rates of infectious complications, 30-day readmissions, and 30-day ED visits. There were zero cases of UTIs or post-prostate biopsy sepsis in the transperineal group in our study. Our study population was also fairly heterogeneous and high-risk for infectious complications with >40% of each cohort having undergone a prior prostate biopsy. Consequently, we add to the limited real-world evidence comparing the safety of transperineal biopsies, particularly without the routine use of antibiotics, to that of the traditional transrectal approach.
The ProBE-PC study and PREVENT trial were recently published, which attempted to directly evaluate infectious and noninfectious complications between patients undergoing transperineal and transrectal prostate biopsies.7,8 These were the first randomized clinical studies directly comparing the two with regard to rates of infectious complications. The ProBE-PC study resulted first and reported no significant differences between the transrectal and transperineal cohorts (2.6% vs. 2.7%) with no cases of sepsis or hospitalizations in either group. These infections were all febrile but did not require intensive care. Similar to our study, the ProBE-PC study utilized a heterogeneous population with the only exclusion criteria being a surgically absent rectum. 100% of patients in the transrectal group received either oral or intramuscular antibiotics while only 1 patient in the transperineal group received antibiotics of any kind. While in the present study the vast majority of patients in the transperineal group did not receive a preoperative bowel preparation, nearly 100% of patients in the transperineal group in the ProBE-PC study did receive a bowel preparation consisting of an enema. The definition of infectious complications within the ProBE-PC study was also particularly broad, encompassing not only sepsis, fevers, and UTIs, but also subjective fevers, phone calls to the office for possible infection, and antibiotic prescriptions by anyone.
The PREVENT trial just recently resulted and also reported no statistically significant differences in infectious complications between the transperineal and transrectal groups—0 cases of infections compared to 1.4% in the transrectal arm (p = 0.059). Contrary to our study, which included all patients who underwent a biopsy, the PREVENT trial excluded patients who previously underwent a prostate biopsy and those who had acute bacterial prostatitis within the past 6 months, limiting the study to a homogenous and relatively low-risk population. The PREVENT trial also utilized a pre-operative rectal culture in order to screen for fluoroquinolone-resistant organisms and ensure targeted antibiotic prophylaxis was administered. Despite the exclusion criteria prohibiting enrollment of some of the higher risk patients into the trial, this study nearly reached statistical significance with a p-value of 0.059, questioning whether broader inclusion criteria or a larger sample size could have yielded results in favor of the transperineal arm.
In the ProBE-PC study and PREVENT trial, the data on hospitalizations and sepsis in the transrectal arms is quite different from what was seen in the present study where we found that 2.3% of patients became septic and 4.02% of patients were readmitted within 30 days. While the ProBE-PC study and PREVENT trials are particularly timely and of significant interest, the low rates of post-prostate biopsy sepsis and hospitalizations within their transrectal cohorts, deviate significantly from what has previously been reported in the literature.2,10–14
Prior to the recent randomized controlled trials, there were multiple observational or single arm studies that attempted to quantify the rate of infectious complications following transperineal and transrectal prostate biopsies. In nearly all of these studies, patients routinely received peri-operative antibiotic prophylaxis. Pepdjonovic et al. prospectively followed 577 patients who underwent a transperineal prostate biopsy and received antimicrobial prophylaxis with cefazolin. They had zero readmissions and one patient who developed prostatitis.15 Tops et al. published data from a European cohort in which they retrospectively analyzed all prostate biopsies done in two hospitals over a 7-year period in which all patients received antibiotic prophylaxis peri-procedurally. They found that patients who underwent a transperineal biopsy had a reduced risk of infectious complications.16 Chen et al. retrospectively compared 212 patients who underwent a transperineal prostate biopsy to 178 patients who underwent a transrectal biopsy. Nearly all patients in both groups received prophylactic antibiotics. A bowel preparation was also performed in both groups. There was a 0% rate of sepsis in the transperineal group compared to 2.2% in the transrectal group.17
With the rise in antimicrobial resistant organisms, the importance of appropriate antibiotic stewardship is paramount. If no significant changes are made, by 2050 antimicrobial resistant organisms will account for 10 million deaths per year, resulting in a global GDP loss of $100.2 trillion.18 As opposed to much of the previously reported data comparing infectious complications in transperineal and transrectal prostate biopsies, in our study we performed nearly all transperineal biopsies without peri-procedural antimicrobial prophylaxis. Castellani et al. published a systematic review and meta-analysis in which they assessed infectious complications in those who underwent a transperineal biopsy with and without antibiotic prophylaxis. The rate of genitourinary infectious was 0.11% in the group receiving antibiotics and 0.31% in the group not receiving antibiotics.19 The recent NORAPP trial randomly assigned patients to either receive peri-procedural antibiotic prophylaxis or not prior to transperineal biopsy. Out of 553 patients, they found that in both groups there were zero infectious complications that required hospitalization.20 Sigle et al. recently published data looking at 184 patients undergoing transperineal biopsy without antibiotic prophylaxis in a European cohort. They reported zero cases of sepsis and two cases of afebrile UTIs.21
Recent studies have begun looking at ways to optimize transrectal biopsies in order to decrease infectious complications while also promoting appropriate antibiotic stewardship. The use of routine pre-biopsy rectal swabs has gained momentum in an effort to tailor antimicrobial prophylaxis prior to biopsy. Tops et al. randomized 1288 patients in a European cohort to either rectal culture-directed antibiotic prophylaxis or standard of care antibiotic prophylaxis prior to biopsy, most commonly with ciprofloxacin. The results indicate that when rectal culture-directed antibiotic prophylaxis was used there was a decrease in the rates of bacteremia within 30 days. Patients who had ciprofloxacin resistant pre-biopsy rectal cultures conferred a 6.2 times increased likelihood of developing an early post-biopsy infectious complication.22
Our study has several limitations beyond its retrospective design. The small sample size and lack of infectious complications in the transperineal group also precludes us from performing a regression analysis. It is also probable that we did not capture all infectious complications and ER/hospital visits as a certain number of patients likely went to other hospitals outside of our network. There were also many patients in the transrectal group who received oral antibiotics before and/or after the procedure in addition to the peri-procedural dose but we were unable to quantify this amount. Lastly, because the decision to perform a bowel preparation was ultimately left to the performing urologist, we still had 35% of patients in the transperineal group receive a bowel preparation.
Current AUA guidelines don’t offer clear recommendations regarding whether urologists should perform transperineal or transrectal prostate biopsies. To date, there has been conflicting data regarding the benefit of transperineal prostate biopsies compared to the traditional transrectal approach. In this study we did not routinely give peri-procedural antibiotic prophylaxis and when compared to the transrectal group in which every patient received antibiotics, there were still significant differences in rates of infectious complications. This is in the setting of infrequent use of a pre-biopsy bowel preparation in a heterogeneous population where >40% of each cohort underwent a prior prostate biopsy. Further prospective studies and high-level randomized trials are needed to assess the potential advantage of the transperineal prostate biopsy compared to both traditional transrectal prostate biopsies and also to patients receiving rectal culture-directed antibiotic prophylaxis prior to transrectal biopsies.
Acknowledgement
All authors would like to thank the Rutgers Cancer Institute of New Jersey.
Funding Statement
The work from the Cancer Institute of New Jersey is supported by a grant from the National Cancer Institute: P30CA072720. Arnav Srivastava is supported by a training grant from the National Cancer Institute: T32CA180984.
Author Contributions
Benjamin J. Lichtbroun: Study design, article writing, and article editing. Mann Patel: Data collection and data analysis. Alexis Consalvo: Data collection and data analysis. Labeeqa Khizir: Data collection and data analysis. Munisa Said: Data collection and data analysis. Austin Chien: Study design Kevin Chua: Article editing. John Pfail: Article editing. Rachel Passarelli: Article writing. Arnav Srivastava: Study design, article writing, article editing. Vignesh T. Packiam: Article editing. David Golombos: Article editing. Sammy Elsamra: Article editing. Thomas L. Jang: Article editing. Saum Ghodoussipour: Study design and article editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The raw data supporting the conclusions of this article will be made available by the authors on request.
Ethics Approval
Ethical approval was obtained from the local Institutional Review Board (IRB No. Pro2022000891).
Conflicts of Interest: Vignesh T. Packiam has acted as a paid consultant for Guidepoint and Valar labs for work outside of the current study. Saum Ghodoussipour has acted as a paid a consultant for Urogen and Janssen for work outside of the current study. Sammy Elsamra has acted as a paid consultant for Intuitiv Surgical Inc for work performed outside of the current study.
References
1. Wei JT, Barocas D, Carlsson S et al. Early detection of prostate cancer: AUA/SUO guideline part I: prostate cancer screening. J Urol 2023;210(1):46–53. [Google Scholar]
2. Nam RK, Saskin R, Lee Y et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 2013;189(1 Suppl):S12–S17. doi:10.1016/j.juro.2012.11.015. [Google Scholar] [CrossRef]
3. Halpern JA, Sedrakyan A, Dinerman B, Hsu WC, Mao J, Hu JC. Indications, utilization and complications following prostate biopsy: New York State analysis. J Urol 2017;197(4):1020–1025. doi:10.1016/j.juro.2016.11.081. [Google Scholar] [CrossRef]
4. Borghesi M, Ahmed H, Nam R et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol 2017;71(3):353–365. doi:10.1016/j.eururo.2016.08.004. [Google Scholar] [CrossRef]
5. Lomas DJ, Ahmed HU. All change in the prostate cancer diagnostic pathway. Nat Rev Clin Oncol 2020;17(6):372–381. doi:10.1038/s41571-020-0332-z. [Google Scholar] [CrossRef]
6. EAU. Prostate Cancer Guidelines. Arnhem, The Netherlands: EAU; 2023. [Google Scholar]
7. Mian BM, Feustel PJ, Aziz A et al. Complications following transrectal and transperineal prostate biopsy: results of the ProBE-PC randomized clinical trial. J Urol 2024;211(2):205–213. doi:10.1097/ju.0000000000003788. [Google Scholar] [CrossRef]
8. Hu JC, Assel M, Allaf ME et al. Transperineal versus transrectal magnetic resonance imaging-targeted and systematic prostate biopsy to prevent infectious complications: the PREVENT randomized trial. Eur Urol 2024;86(1):61–68. doi:10.1016/j.eururo.2023.12.015. [Google Scholar] [CrossRef]
9. Udeh EI, Amu OC, Nnabugwu II, Ozoemena O. Transperineal versus transrectal prostate biopsy: our findings in a tertiary health institution. Niger J Clin Pract 2015;18(1):110–114. doi:10.4103/1119-3077.146991. [Google Scholar] [CrossRef]
10. Forsvall A, Jönsson H, Wagenius M, Bratt O, Linder A. Rate and characteristics of infection after transrectal prostate biopsy: a retrospective observational study. Scand J Urol 2021;55(4):317–323. doi:10.1080/21681805.2021.1933169. [Google Scholar] [CrossRef]
11. Gross MD, Alshak MN, Shoag JE et al. Healthcare costs of post-prostate biopsy sepsis. Urology 2019;133(Suppl 3):11–15. doi:10.1016/j.urology.2019.06.011. [Google Scholar] [CrossRef]
12. Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol 2012;62(3):453–459. doi:10.1016/j.eururo.2012.04.044. [Google Scholar] [CrossRef]
13. Carmignani L, Picozzi S, Spinelli M et al. Bacterial sepsis following prostatic biopsy. Int Urol Nephrol 2012;44(4):1055–1063. doi:10.1007/s11255-012-0145-9. [Google Scholar] [CrossRef]
14. Liss MA, Ehdaie B, Loeb S et al. An update of the American urological association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol 2017;198(2):329–334. doi:10.1016/j.juro.2017.01.103. [Google Scholar] [CrossRef]
15. Pepdjonovic L, Tan GH, Huang S et al. Zero hospital admissions for infection after 577 transperineal prostate biopsies using single-dose cephazolin prophylaxis. World J Urol 2017;35(8):1199–1203. doi:10.1007/s00345-016-1985-1. [Google Scholar] [CrossRef]
16. Tops SCM, Grootenhuis JGA, Derksen AM et al. The effect of different types of prostate biopsy techniques on post-biopsy infectious complications. J Urol 2022;208(1):109–118. doi:10.1097/JU.0000000000002497. [Google Scholar] [CrossRef]
17. Chen KW, Pek G, Qiao Y et al. Comparing outcomes of transperineal to transrectal prostate biopsies performed under local anaesthesia. BJUI Compass 2021;3(3):197–204. doi:10.1002/bco2.112. [Google Scholar] [CrossRef]
18. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019;12:3903–3910. doi:10.2147/IDR.S234610. [Google Scholar] [CrossRef]
19. Castellani D, Pirola GM, Law YXT et al. Infection rate after transperineal prostate biopsy with and without prophylactic antibiotics: results from a systematic review and meta-analysis of comparative studies. J Urol 2022;207(1):25–34. doi:10.1097/JU.0000000000002251. [Google Scholar] [CrossRef]
20. Jacewicz M, Günzel K, Rud E et al. Antibiotic prophylaxis versus no antibiotic prophylaxis in transperineal prostate biopsies (NORAPP): a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2022;22(10):1465–1471. doi:10.1016/S1473-3099(22)00373-5. [Google Scholar] [CrossRef]
21. Sigle A, Suarez-Ibarrola R, Pudimat M et al. Safety and side effects of transperineal prostate biopsy without antibiotic prophylaxis. Urol Oncol 2021;39(11):e1–e5. doi:10.1016/j.urolonc.2021.02.016. [Google Scholar] [CrossRef]
22. Tops SCM, Kolwijck E, Koldewijn EL et al. Rectal culture-based versus empirical antibiotic prophylaxis to prevent infectious complications in men undergoing transrectal prostate biopsy: a randomized, nonblinded multicenter trial. Clin Infect Dis 2023;76(7):1188–1196. doi:10.1093/cid/ciac913. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools