 Open Access
Open Access
ARTICLE
A practical approach to the difficult urethral catheterization for urology trainees
1 Department of Urology, Dalhousie University, Halifax, NS B3H 4R2, Canada
2 Division of Pediatric Urology, IWK Health Centre, Dalhousie University, Halifax, NS B3H 4R2, Canada
* Corresponding Author: Wyatt MacNevin. Email:
Canadian Journal of Urology 2025, 32(1), 5-13. https://doi.org/10.32604/cju.2025.064697
Received 05 October 2024; Accepted 31 December 2024; Issue published 20 March 2025
Abstract
Urethral catheterization is an important skill to develop as consultations for “difficult catheterization” are common in practice. Developing a broad approach to difficult urethral catheterization is crucial to improve trainee success rates. Strategies and techniques to improve catheterization success are often passed down and shared between trainees without formal documentation or dissemination of techniques. Herein, we present a framework for difficult urethral catheterization based on clinical history and patient examination, while also providing additional techniques and troubleshooting to overcome common challenges with urethral catheterization in adult and pediatric patients.Keywords
Urethral catheterization is a common procedure to facilitate bladder drainage, monitor accurate urine output, and to manage incontinence.1 Although basic urethral catheterization techniques are taught in medical and nursing school, urology consultation is often required for more challenging cases.
Having a framework is crucial when approaching a difficult urethral catheterization. After identifying patient/equipment factors that hinder catheterization success, different techniques or approaches can be used to increase success rates. Unfortunately, these techniques are mostly undocumented and informally taught during Urology training. Herein, we present a practical approach to difficult urethral catheterization and present techniques and strategies for safe catheterization. These techniques and strategies were developed by personal experience and literature review.
General patient assessment, catheter selection, and technique
Determining the appropriateness of urethral catheterization is important, such as: placement for prolonged surgery/procedures, acute urinary retention, to facilitate wound healing, or for accurate urine output measurement in critically-ill patients.1,2 Otherwise, the use of urinals, clean-intermittent-catheterization, condom catheters, or incontinence pads are less invasive and likely equally effective strategies for urinary management.2 Of note, in suspected urethral injury, characterization with retrograde urethrogram may be required before determining the appropriateness of urethral vs. suprapubic catheterization.1,2
Clinical history and examination will help identify whether the challenging catheterization is due to difficult urethral access or difficult passage and may influence initial catheter selection (Table 1). Prior urethral/prostate surgery, trauma, pelvic radiation, lichen sclerosis, or sexually transmitted infections may indicate urethral stricture disease or bladder neck contracture.3,4 Obstructive voiding symptoms, elevated prostate specific antigen, or enlarged prostate on digital rectal exam and/or cross-sectional imaging, may indicate prostatic enlargement. With suspected prostatic enlargement, initial catheter selection should be an 18 Fr coudé catheter.5 For urethral stricture disease or bladder neck contracture, a 12–14 Fr straight catheter is preferable.3 Silicone catheters may also be used with care in patients with suspected urethral strictures as they can be passed more easily through narrow urethral segments due to their rigidity. For patients with a vulva, initial catheterization with a 14–16 Fr straight catheter is often successful once patient positioning has been optimized (Figure 1). Importantly, if urethral catheterization cannot be achieved with the techniques discussed in this paper, suprapubic cystostomy should be performed with ultrasound-guidance or by interventional radiology. A 18–20 g spinal needle can also be inserted 2 finger-breadths above the pubic symphysis to decompress the bladder and temporize patients in severe discomfort from urinary retention.

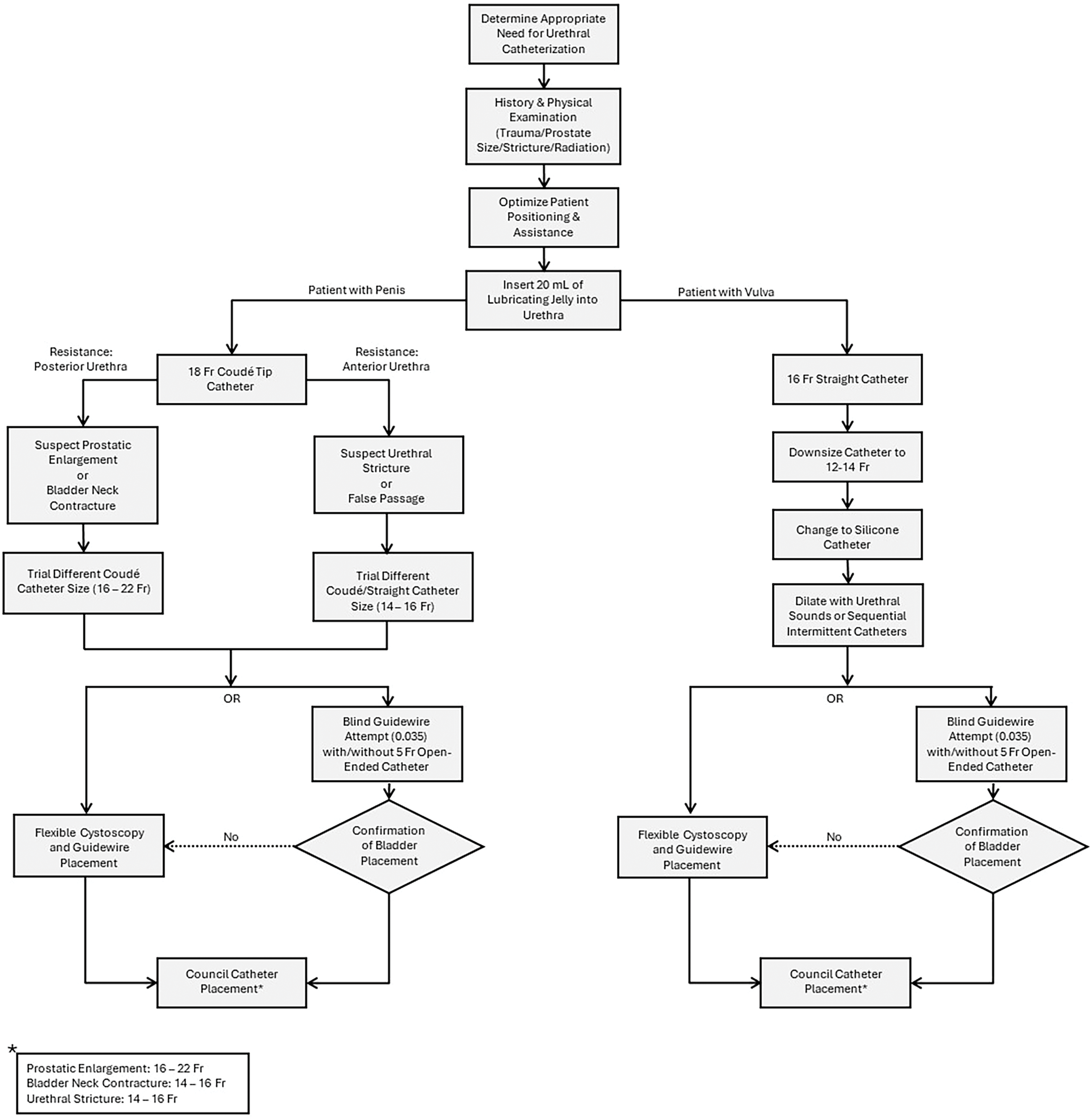
FIGURE 1. Practical approach to difficult urethral catheterization
Patient positioning and difficult urethral access
For patients with a penis, positioning is less important as visualization of the urethral meatus is easily obtained, unless the patient has a significant suprapubic fat pad or buried penis. The supine position is the most effective position. Lateral decubitus can be used if the patient cannot tolerate supine positioning. Although blind passage of a catheter may be a successful in patients with difficult urethral access/visualization, the use of a speculum to visualize and locate the urinary meatus or cystoscopy may be necessary.6 Downwards pre-pubic pressure may also aid in visualization of the urethral meatus. With the aid of additional personnel, catheter placement can be facilitated in this manner.
Phimosis or edema/anasarca can also cause difficult urethral access. In patients with phimosis, performing a dorsal slit/circumcision to facilitate catheterization is the most definitive approach.5 Alternatively, using local anesthesia and spreading the phimotic tissue with a surgical instrument (e.g., Snap/hemostat) may allow for visualization of the urethral meatus. Blind insertion of a 5 Fr open-ended ureteric catheter or smaller (8–10 Fr) in-and-out catheter through the stenotic foreskin and into the bladder, can be done for temporizing bladder decompression. In a patient with anasarca or penile edema, minimizing excess lubrication and applying prolonged pressure to reduce the edema can greatly improve catheterization success. This can also be achieved by temporarily applying a compressive dressing circumferentially to the penis. The use of gauze to improve penile grip with the non-dominant hand can improve ease of catheter insertion. If this fails, under sterile technique, a small incision and drainage of the fluid can help reduce the redundant penile skin to improve visualization.
Optimizing patient positioning is often all that is required for catheterizing patients with vulvas. To expose the urethral meatus, the patient should be in lithotomy or in the “frog” position with the hips and knees partially flexed, heels together, and hips abducted. Additional personnel may be required to guide/support the patient into this position. Additionally, use of a pillow, towel, or the patient’s hands under the lower-back is helpful for realigning the pelvis to improve visualization. Once positioning is optimized, the thumb, middle, and index fingers of the non-dominant hand should gently separate the labia while pulling upwards to expose the urethral meatus. For patients with vaginal atrophy, there may be intravaginal retraction of the urethral meatus in which this technique may be necessary. Additionally, if the urethra can be palpated along the anterior vaginal wall, a finger can be inserted into the vagina to guide catheter placement. If a previous catheterization attempt resulted in vaginal catheter placement, leaving the catheter can aid in urethral placement during subsequent attempts. A coudé catheter can also be used in this scenario with the upward curvature deflected along the anterior vaginal wall and into the urethra. If these strategies are not sufficient, cystoscopy-assisted catheter placement may be necessary.
Standard catheterization technique
Once positioning has been optimized and catheter type and size has been selected, 20 cc’s of 2% lidocaine lubricant instilled per urethra is recommended to improve discomfort while also dilating the urethra.7,8 For patients with a penis, grasp the penis at the corona between the middle and ring fingers of the non-dominant hand, place the penis on upwards stretch, slowly instill the lubricant, then pinch the meatus closed to minimize lubricant leakage (Figure 2). Once the lubricant has been instilled, with the penis on either upwards stretch or stretched towards the patient’s feet, a catheter should be slowly advanced into the bladder. If using a coudé catheter, care should be taken to confirm the tip is deflected upwards by ensuring the inflation port/rubber nubbin is at the 12 o’clock position (Figure 2). The catheter should be advanced completely to the hub to avoid balloon inflation in the prostate or urethra.9 If the catheter does not stay in position once released at the hub, the catheter may not be properly placed and may need repositioning.9 Additionally, if patients feel discomfort during catheter balloon inflation, this supports improper catheter placement in the prostate or urethra. To verify catheter position before inflating the balloon, the lidocaine jelly plunger can be depressed, inserted into the catheter outflow port and released; suctioning urine from the bladder (Figure 3). This can also be done by using an appropriately-sized syringe in the catheter outflow channel. Once the balloon is inflated, easy retraction of the catheter to the bladder neck further supports successful placement. If uncertainty persists, bedside ultrasound can be performed to visualize the catheter/balloon in the bladder.
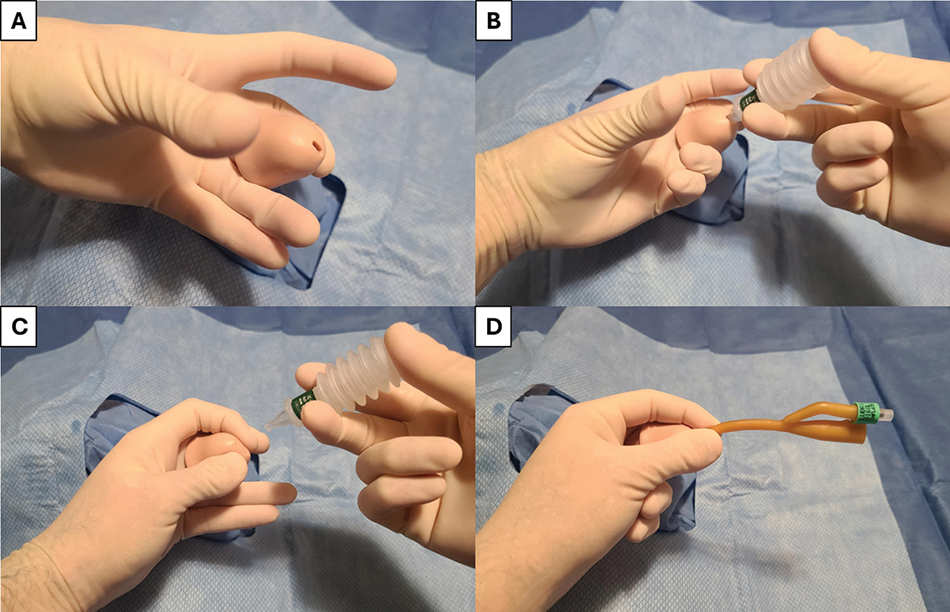
FIGURE 2. Standard catheterization technique—(A) Penis is grasped between middle and ring fingers of non-dominant hand, (B) lidocaine jelly is instilled slowly and gently per urethra, (C) After instillation of lidocaine jelly, urethral meatus is gently pinched closed to reduce leakage of lubricant, (D) Catheter is gently advanced per urethra; ensuring balloon instillation port is at 12 o’clock position for coudé catheters
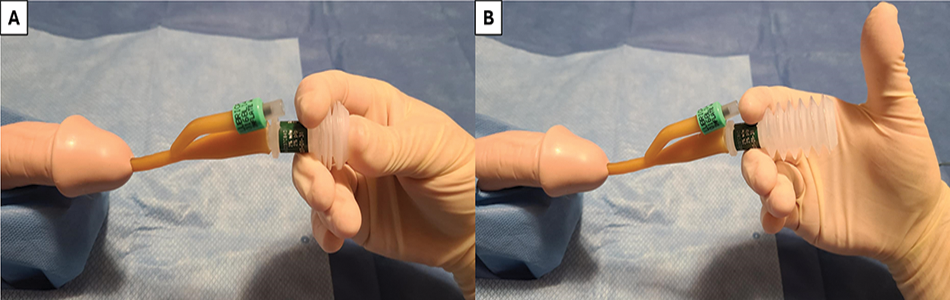
FIGURE 3. Confirmation of placement—One technique to support appropriate catheter placement is shown: (A) Depression of the lidocaine jelly applicator is performed and placed into catheter outflow, (B) With proper placement, urine will be suctioned into the applicator once pressure is removed
Guidewire use to facilitate catheter passage is an integral skill for approaching difficult catheterizations.10 Guidewire placement into the bladder can be done blindly or under direct vision with flexible cystoscopy. Guidewire types include PTFE-coated straight, pure hydrophilic wires, hybrid PTFE-nitinol wires with a hydrophilic tip, and stiff Amplatz wires—each with particular advantages.
Blind guidewire placement can be done using a purely hydrophilic coated/hybrid guidewire by gently advancing the guidewire into the urethral meatus and into the bladder. Confirmation of bladder entry can be done either fluoroscopically, with ultrasound, or by inserting a 5 Fr open-ended ureteric catheter over the guidewire with visualization of urine backflow (Figure 4). Attaching a 10 cc syringe to the ureteric catheter and flushing out the lubricant will often allow for urine backflow. Guidewire exchange can be performed through the open-ended catheter once bladder access is obtained. Additionally, blind open-ended catheter placement can be done initially, with subsequent placement of the guidewire through the open-ended catheter. If blind guidewire placement is unsuccessful, it usually presents with return of the wire back through the urethral meatus (for pure hydrophilic wires) or soft deflection of the penis as the wire is advanced (for hybrid wires).11
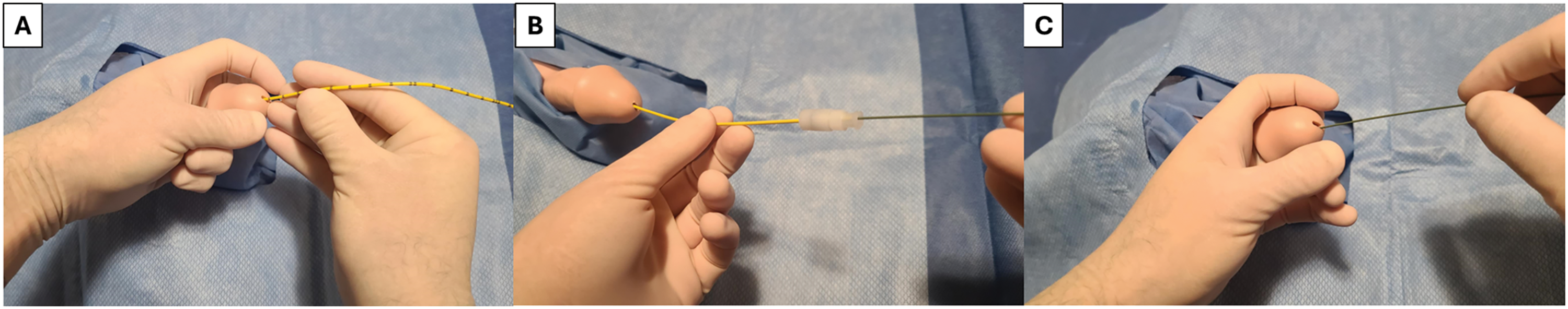
FIGURE 4. Open-ended ureteric catheter and guidewire use—(A) 5 Fr open-ended ureteric catheter is gently advanced per urethra until urine is seen draining, alternatively, a 10 cc syringe can be attached to the open-ended ureteric catheter and flushed to allow for urine backflow (B) Guidewire is passed through the open-ended catheter to gain access to the bladder, (C) Open-ended catheter is backloaded, leaving the guidewire in place to facilitate passage of catheter over guidewire. Alternatively, the guidewire can be passed first per urethra, with open-ended catheter placed over the guidewire to confirm location in the bladder. If the guidewire is being placed blindly, care should be taken to landmark the guidewire at ~45 cm, and once this location is inserted into the urethra, it is supportive that the wire has entered the bladder
For patients with suspected urethral strictures, soft guidewires are recommended to navigate the narrowed urethral segment without causing trauma. Hybrid guidewires are recommended for patients with false passages to gently cannulate the lumen while having enough rigidity to pass through damaged urethral tissue. Stiff guidewires are recommended once bladder access is obtained through wire exchange via an open-ended catheter. Urethral dilatation should be done over a stiff guidewire as dilatation over a soft wire can lead to urethral/bladder injury or wire damage.
Once a guidewire is placed, a Council-tip catheter can be advanced into the bladder over the guidewire (Figure 5). Lubricant should be instilled through the catheter and along the guidewire to reduce friction. When a Council-tip catheter is not available, the Blitz technique, can be utilized to pass a non-Council-tip catheter over a guidewire (Figure 6).12 This technique involves placement of a 16–18 gauge IV catheter with needle through the distal drainage hole of a urethral catheter and then out of the catheter tip. The needle is removed and the guidewire is passed through the IV catheter/needle and directed inside the drainage lumen. Alternatively, the distal tip of a non-Council-tip catheter can be cut across or crosshatched (with an 11-blade) distal to the balloon to allow a guidewire to be inserted into the catheter lumen (Figure 7). Slightly retracting the guidewire while advancing the council catheter can be helpful if too much guidewire is buckling in the bladder.
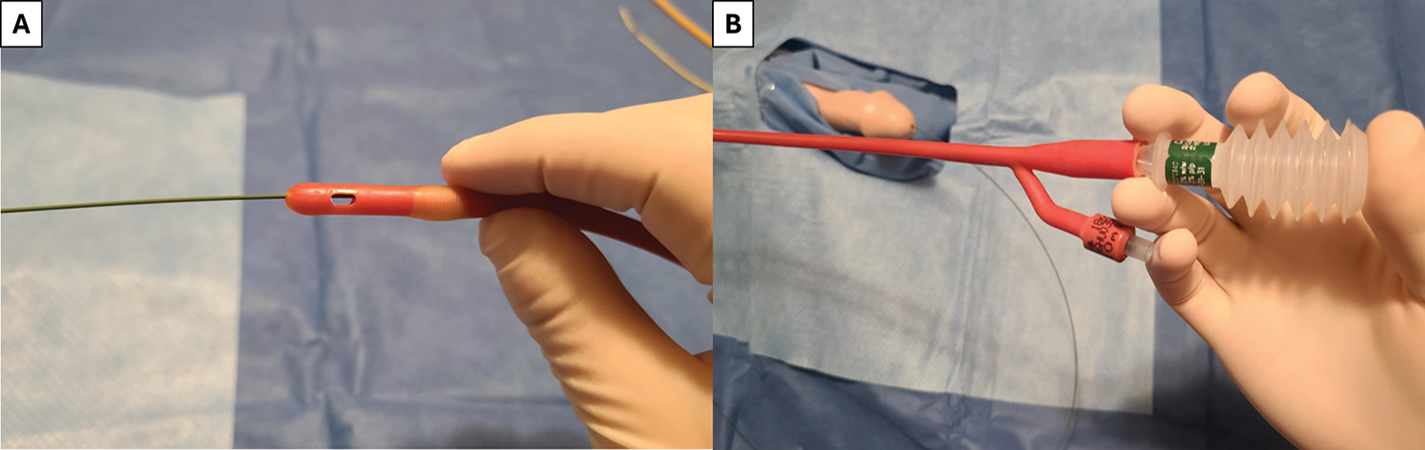
FIGURE 5. Guidewire-assisted catheter placement—(A) Council-tip catheter is used with accessible lumen for passage of the guidewire through the catheter, (B) Lubricant jelly is instilled in the catheter inner lumen to reduce friction on wire while passing the catheter into the bladder
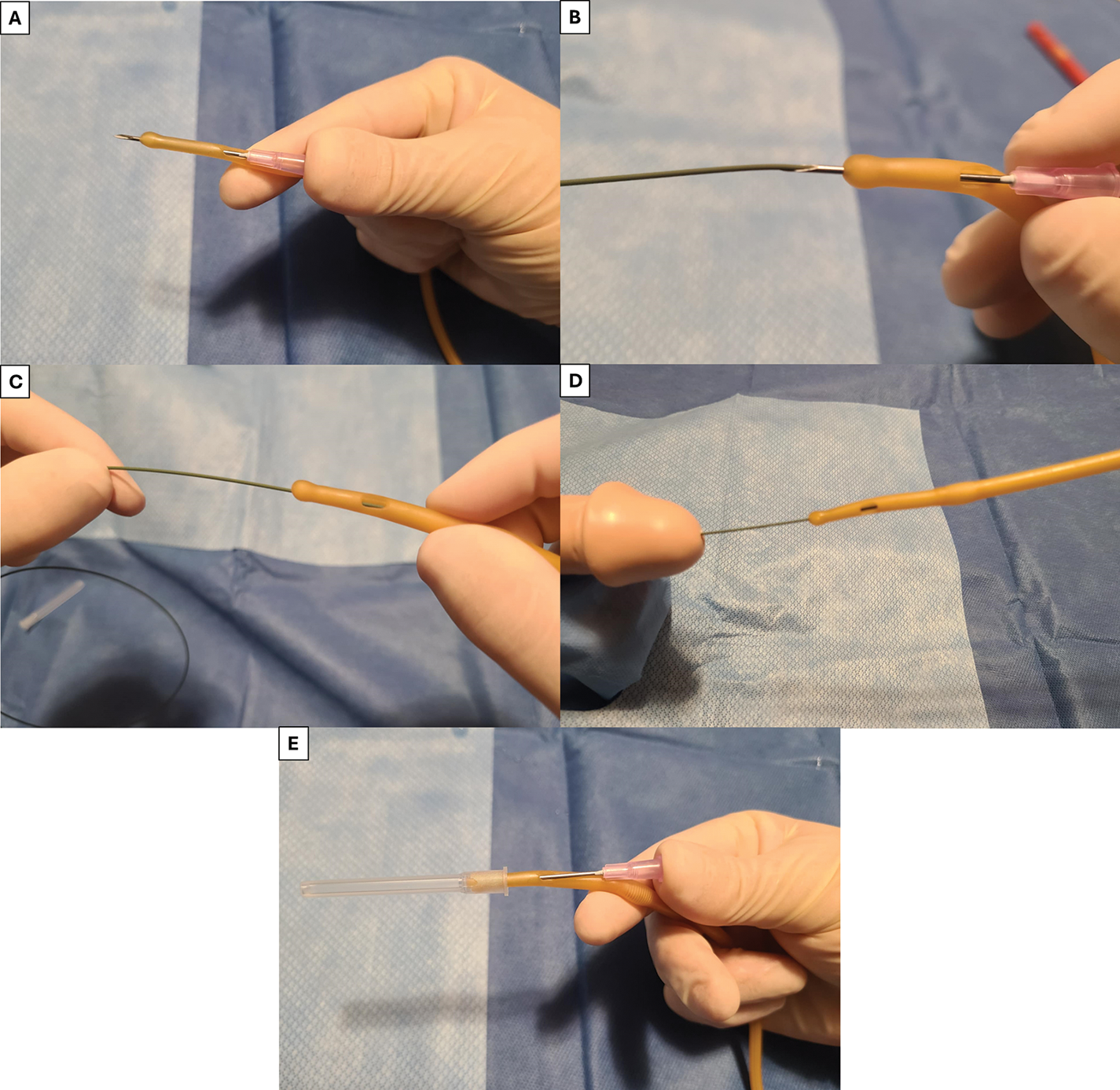
FIGURE 6. Blitz technique—(A) IV needle is inserted through distal side port of catheter and out of the tip, (B) Distal end of the guidewire is then passed through the IV needle opening, (C) IV needle is pulled backwards out of the catheter and guidewire is positioned within the inner lumen of the catheter, (D) Catheter is then advanced over the wire, (E) Alternative technique recommended by the authors using the IV needle cover to ensure needle/wire is centrally located in the catheter while protecting the user from the needle point. Alternatively, an angiocath IV catheter/needle can be used, with the needle withdrawn after puncturing the urethral catheter leaving the IV catheter in place—to reduce risk of operator-injury
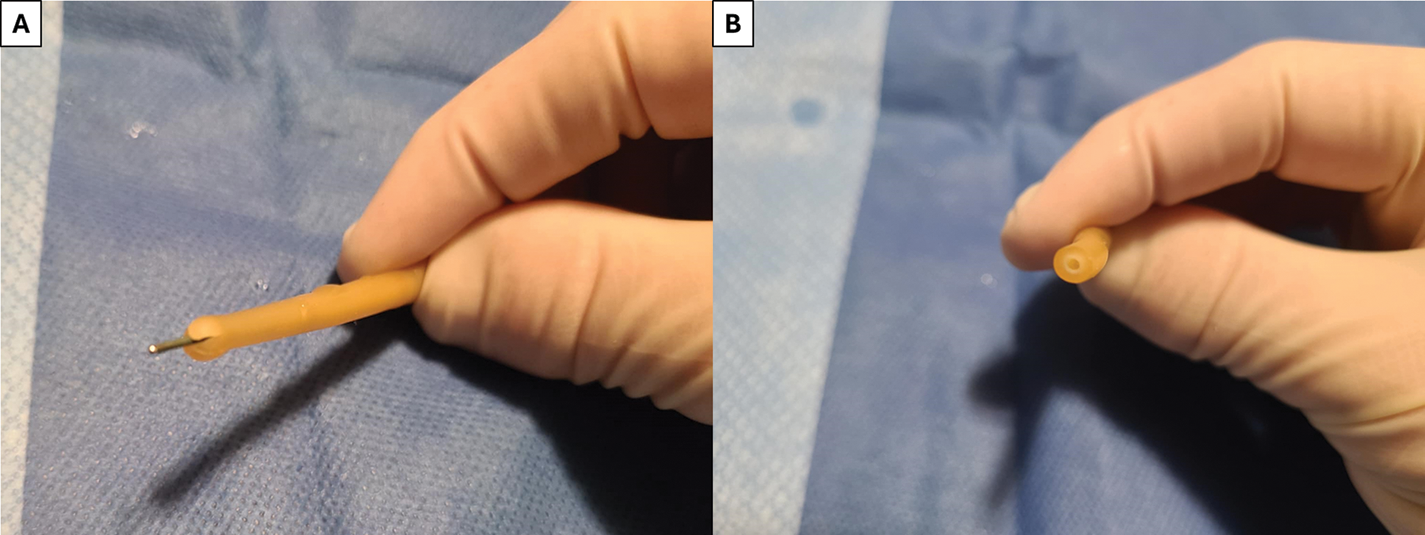
FIGURE 7. Alternative techniques to pass the guidewire through the catheter—(A) Using scissors or a scalpel, the distal tip of the catheter can be cut into an “X” to pass the guidewire into the inner lumen, (B) Distal end of the catheter can be cut across—safety must be taken to not cut into the balloon
Once the urethral meatus has been visualized, catheterization can be difficult due to urethral or prostatic obstruction. These situations are commonly seen in patients with a penis due to urethral length and prostatic hyperplasia. Localizing the urethral obstruction can be estimated based on the length of catheter inserted before meeting resistance, or palpation of the ventral penile surface as the catheter is inserted to the level of resistance. This can differentiate urethral stricture disease vs. prostatic obstruction or bladder neck pathology. For patients with a vulva, urethral obstruction occurs primarily with urethral stenosis. This is commonly seen when urethral visualization is obtained but the catheter cannot pass through the urethra comfortably.
Urethral stricture/stenosis or bladder neck contracture
If the stricture/stenosis is located in the anterior urethra, trial placement of a straight catheter (14–16 Fr in patients with a penis, 12–14 Fr in patients with a vulva) may be successful in navigating through the narrowing. Filiforms and followers can be utilized to dilate the stenotic or contracted urethral segment. Additionally, at the level of the obstruction, 40–60 cc’s of normal saline can be briskly instilled through a catheter while simultaneously advancing the catheter to attempt to dilate and traverse passed the obstruction (Figure 8).8 If unsuccessful, blind guidewire placement and/or a 5 Fr open-ended catheter can be attempted to facilitate catheter placement over the guidewire. If this is unsuccessful, cystoscopy should be performed for visualization of the stricture or false passage and safe placement of a catheter over a guidewire. For severe stricture disease, a rigid ureteroscope or pediatric cystoscope can be used for visualization and guidewire placement. Dilatation over the guidewire can then be carried out using S-curve dilators if necessary. Dilation should be performed to at least 2 Fr greater than the size of the intended catheter to be placed.

FIGURE 8. Additional techniques—(A,B) Guidewire is inserted into small diameter catheter and clamped in place to provide additional stiffness and support, (C) 60 cc syringe is attached to the catheter and depressed at the level of obstruction to facilitate passage of the catheter
Additional techniques include clamping a guidewire inside a smaller diameter catheter to improve stiffness for advancement (Figure 8). A balloon dilator can also be used once a guidewire has been placed to dilate the stricture.
For patients with a vulva and urethral stricture/stenosis, down-sizing the catheter or using a stiffer catheter (silicone) is usually effective. Occasionally, urethral sounding may be required before catheter placement. Alternatively, sequential passing of silicone intermittent-catheters of increasing size can be done to dilate the urethra.
For patients with suspected prostatic hyperplasia, a 16–20 Fr coudé catheter should be used to guide the catheter over the enlarged prostate tissue and into the bladder. Straight catheters may cause the creation of a false passage in these patients as the catheter will not be able to curve along the urethra. Adjunctive techniques include palpating the ventral surface of the urethra as the catheter is being passed, and adding perineal pressure at the approximate level of the bulbar urethra to help push the catheter anteriorly. If use of a coudé catheter is unsuccessful, catheter placement over a wire or flexible cystoscopy may be required.
Flexible cystoscopy/urethroscopy
For difficult catheterizations, cystoscopy-assisted catheter placement is preferred as it is the safest option and allows for urethra and bladder visualization. This must be considered alongside the cost of disposable cystoscopes, cleaning and processing costs, and cystoscope availability. For patients with prostatic enlargement, a flexible cystoscope can usually be easily navigated into the bladder with a guidewire placed and the scope backloaded. For patients with urethral strictures, the cystoscope can be advanced to the level of urethral narrowing and either gently passed through the stricture, or a guidewire can be passed under direct vision to facilitate dilatation or catheter placement. Flexible cystoscopy is most valuable for patients with false passages as the urethral injury can be seen and the true urethral lumen can be identified. Commonly, urethral injuries from unsuccessful catheter placement occurs at the bulbar and posterior urethra.5 Traumatic false passages will be lateral or posterior with jagged edges whereas the true urethral lumen is often located anteriorly. When attempting to locate the true lumen using flexible cystoscopy, the scope should be advanced to the level of trauma and the scope should be deflected anteriorly to locate the true lumen, once advanced 1–2 mm, the scope should be withdrawn to allow for re-establishment of visibility. Increased pressure on the irrigation bag may be required for visibility in situations of urethral bleeding. Gentle guidewire advancement often indicates successful localization of the true lumen.
The pediatric population poses unique challenges as the level of patient cooperativity can add complexity. Newborns and infants can often be soothed during the procedure in a calm environment with adjuncts such as oral sucrose. However, toddlers, children, and even teenagers may become extremely resistant, therefore consideration for sedation may be beneficial. For patients with a vulva, utilizing the frog position and grasping each labia majora and pulling the tissue gently toward yourself can expose the urethra and reduce the likelihood of catheter misplacement. A coudé catheter can also be used for patients with an anteriorly deviated urethra. In pediatric patients with a penis, the foreskin can be tight and often blind placement is necessary. General principles of downward foreskin traction to expose the meatus, followed by penile stretch and a relaxed patient to facilitate passage through the sphincter apply. For complex patients (e.g., Urinary diversions and reconstructions), catheterization is best managed by pediatric urologists.
Having appropriately sized catheters is crucial. Catheter size selection is often based on experience, with 6 Fr being the smallest catheter with a balloon and the smallest coudé catheter being 8 Fr (Figure 9). However, estimating initial catheter size based on patient weight (up to 30 kg) can be approximated by13:
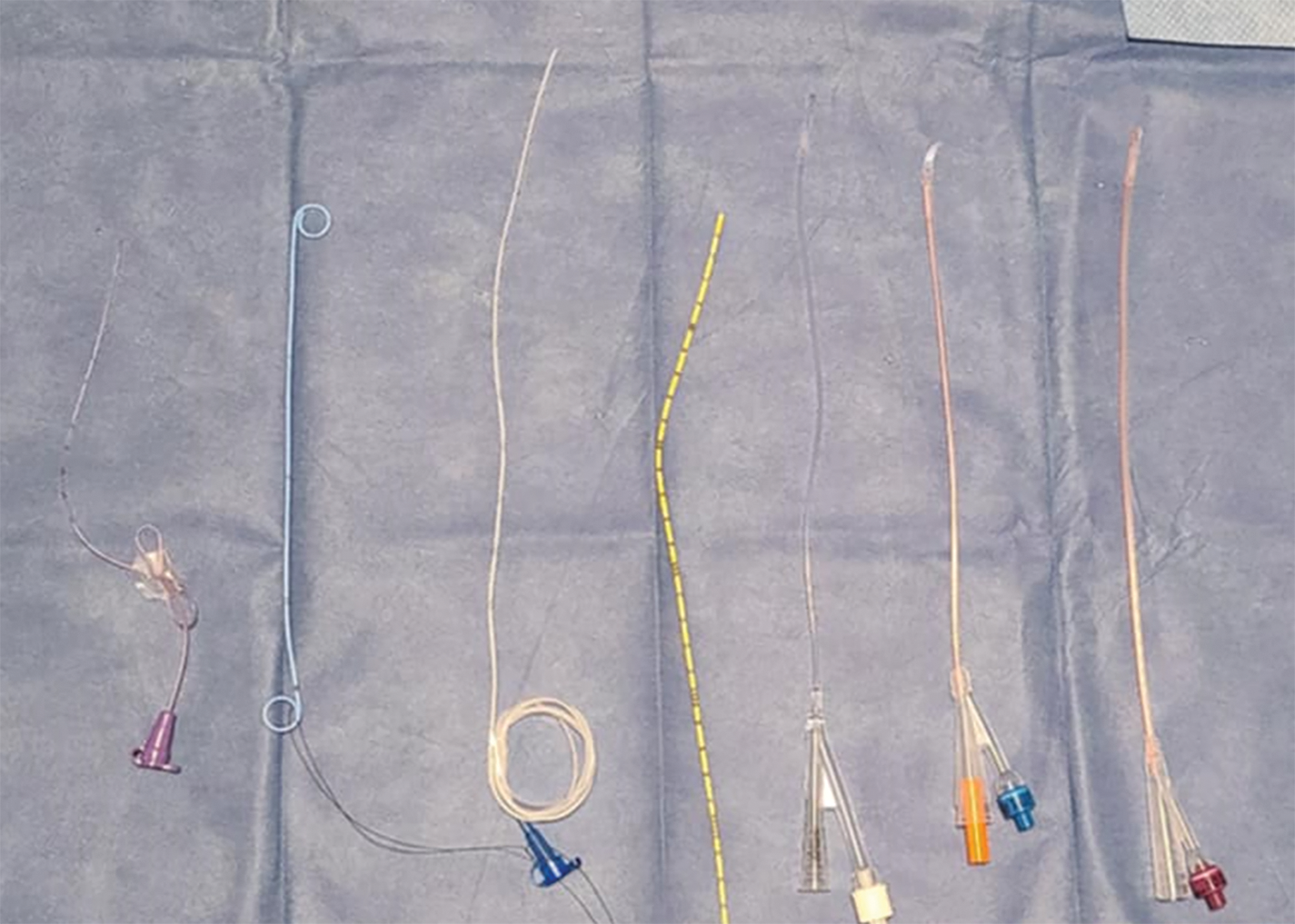
FIGURE 9. Pediatric catheterization options—(Left to Right): 3.5 Fr Feeding Tube, 4.8 Fr Double-J Ureteric Stent, 5 Fr Feeding Tube, 5 Fr Open-Ended Ureteric Catheter, 6 Fr Pediatric Silicone Catheter, 8 Fr Silicone Coudé Tip Pediatric Catheter, 10 Fr Open-Ended Silicone Pediatric Catheter
For newborns and pre-term infants, feeding tubes, which range from 3.5–8 Fr, can be fashioned into urethral stents and taped into position. Although effective, they run the risk of coiling/knotting if advanced to far into the bladder. We recommend advancing only 3–4 cm passed the point where urine drains. To mitigate the risk of knotting, placement of double-J ureteral stents per urethra with advancement over a guidewire aided by ultrasound guidance is a safe alternative.14 This is a preferred method due to the possible presence of posterior urethral valves.
In infant patients with and failed urethral catheterization, suprapubic aspiration can be used to obtain bladder drainage and temporize the patient until transfer to pediatric urology for definitive management. If the pediatric patient is at risk for urine re-accumulation during transfer, a suprapubic double-J stent can then be placed for ongoing drainage.15
Having a systematic approach to difficult urethral catheterization is important to improve success. First, start with identifying appropriate indications for urethral catheterization vs. other urinary management options. A urologic-focused history, review of imaging, and physical exam should be performed in conjunction with obtaining knowledge of prior attempted catheter types and sizes, location of obstruction felt, and techniques attempted. Patient positioning should be optimized, and assistance from additional healthcare personnel enlisted prior to attempting catheterization.
After sterile preparation, instillation of lubrication jelly per urethra should be done to minimize patient discomfort and to gently dilate the urethra.7 Initial catheter selection should be done based on previous attempts, clinical history, and physical examination. For patients with a penis, initial attempt with an 18 Fr coudé catheter is recommended, and if unsuccessful, subsequent attempts should be guided based on the level and cause of suspected obstruction. For patients with a vulva, a 16 Fr straight catheter can be initially trialled, with subsequent attempts with silicone or coudé catheters being used if necessary.
If initial catheterization attempts are unsuccessful, flexible cystoscopy or blind guidewire placement is recommended. Cystoscopic-assisted guidewire placement is recommended as it is diagnostic, safe, and provides visualization of the urethra and bladder, although this must be considered alongside cystoscope availability, cost, and resources. After confirming successful guidewire placement, S-dilation can be performed if required, prior to catheter placement in the bladder. Importantly, if urethral catheterization is unable to be performed, suprapubic cystostomy may be required.
The approach to difficult urethral catheterization is often developed based on personal experience with very little existing in the literature to aid in skill development and learning. This paper presents a practical framework to improve catheterization success, while also sharing advanced catheterization techniques using guidewires, dilators, and cystoscopy. Additionally, the topic of pediatric catheterization is presented to help provide information for addressing difficult catheterization in this complex patient group.
Having a practical approach to difficult urethral catheterization is imperative to minimize patient discomfort and improve catheterization success. We recommend our proposed framework for approaching difficult urethral catheterization in combination with adjunctive techniques, guidewire use, cystoscopy/urethroscopy, and dilators when necessary.
Acknowledgement
Not applicable.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
Wyatt MacNevin—contributed to conceptualization, methodology, writing, figure preparation, review, and editing. Daniel T. Keefe—contributed to conceptualization, methodology, writing, review, supervision. Karen Milford—contributed to methodology, review, and editing. Nicholas R. Paterson—contributed to conceptualization, methodology, figure preparation, review, editing, and supervision. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
Not applicable.
Ethics Approval
Formal ethics review was waived for this project after review from the institutional research ethics board.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Jansen IAV, Hopmans TEM, Wille JC, van den Broek PJ, van der Kooi TII, van Benthem BHB. Appropriate use of indwelling urethra catheters in hospitalized patients: results of a multicentre prevalence study. BMC Urol 2012;12(1):25. doi:10.1186/1471-2490-12-25. [Google Scholar] [CrossRef]
2. Wagner KR, Bird ET, Scott Coffield K. Urinary catheterization: a paradigm shift in difficult urinary catheterization. Curr Urol Rep 2016;17(11):82. doi:10.1007/s11934-016-0641-z. [Google Scholar] [CrossRef]
3. King C, Rourke KF. Urethral stricture is frequently a morbid condition: incidence and factors associated with complications related to urethral stricture. Urology 2019;132(Suppl 4A):189–194. doi:10.1016/j.urology.2019.07.013. [Google Scholar] [CrossRef]
4. Lazzeri M, Sansalone S, Guazzoni G, Barbagli G. Incidence, causes, and complications of urethral stricture disease. Eur Urol Suppl 2016;15(1):2–6. doi:10.1016/j.eursup.2015.10.002. [Google Scholar] [CrossRef]
5. Ghaffary C, Yohannes A, Villanueva C, Leslie SW. A practical approach to difficult urinary catheterizations. Curr Urol Rep 2013;14(6):565–579. doi:10.1007/s11934-013-0364-3. [Google Scholar] [CrossRef]
6. Hewes JC, Kelly J, Hashemi M. Buried penis in super obesity: a technique for urethral catheterization under direct vision. Surg Obes Relat Dis 2011;7(3):332. doi:10.1016/j.soard.2011.01.036. [Google Scholar] [CrossRef]
7. Siderias J, Gaudio F, Singer AJ. Comparison of topical anesthetics and lubricants prior to urethral catheterization in males: a randomized controlled trial. Acad Emerg Med 2004;11(6):703–706. [Google Scholar]
8. Villanueva C, Hemstreet III GP. Difficult male urethral catheterization: a review of different approaches. Int Braz J Urol 2008;34(4):401–412. doi:10.1590/s1677-55382008000400002. [Google Scholar] [CrossRef]
9. Kashefi C, Messer K, Barden R, Sexton C, Parsons JK. Incidence and prevention of iatrogenic urethral injuries. J Urol 2008;179(6):2254–2258. doi:10.1016/j.juro.2008.01.108. [Google Scholar] [CrossRef]
10. Villanueva C, Hemstreet GP 3rd. The approach to the difficult urethral catheterization among urology residents in the United States. Int Braz J Urol 2010;36(6):710–717. doi:10.1590/s1677-55382010000600009. [Google Scholar] [CrossRef]
11. Luangkhot R, Cheng E, Yuan M et al. A simple guidewire and angiocatheter technique for urinary catheter placement. JAAPA 2019;32(8):39–42. doi:10.1097/01.JAA.0000569800.52435.71. [Google Scholar] [CrossRef]
12. Blitz BF. A simple method using hydrophilic guide wires for the difficult urethral catheterization. Urology 1995;46(1):99–100. doi:10.1016/S0090-4295(99)80169-1. [Google Scholar] [CrossRef]
13. Kopač M. Formula estimation of appropriate urinary catheter size in children. J Pediatr Intensive Care 2013;2(4):177–180. doi:10.3233/pic-13069. [Google Scholar] [CrossRef]
14. Penna FJ, Bowlin P, Alyami F, Bägli DJ, Koyle MA, Lorenzo AJ. Novel strategy for temporary decompression of the lower urinary tract in neonates using a ureteral stent. J Urol 2015;194(4):1086–1090. doi:10.1016/j.juro.2015.04.102. [Google Scholar] [CrossRef]
15. Keefe DT, Andrioli V, Leonard MP. Techniques-temporary suprapubic diversion in a septic male infant using double-J stent: indications and surgical technique. Can Urol Assoc J 2018;12(7):E362–E363. doi:10.5489/cuaj.5018. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools