 Open Access
Open Access
CASE REPORT
Diagnosis and management of a rare paratesticular venous malformation in a pediatric patient
1 Department of Urology, Boston Medical Center, Boston, MA 02118, USA
2 Department of Urology, Boston Children’s Hospital, Boston, MA 02115, USA
3 Department of Anatomic Pathology, Boston Children’s Hospital, Boston, MA 02115, USA
4 Department of Radiology, Boston Children’s Hospital, Boston, MA 02115, USA
* Corresponding Author: H. Thaker. Email:
# These authors contributed equally to this work
Canadian Journal of Urology 2025, 32(1), 43-46. https://doi.org/10.32604/cju.2025.064688
Received 30 July 2024; Accepted 23 January 2025; Issue published 20 March 2025
Abstract
A 14-year-old presented with an asymptomatic left testicular mass after a brief episode of pain. Examination showed a non-tender left testis that was significantly larger than the right. Ultrasound revealed a 4.5-cm avascular mass and an absence of normal testicular parenchyma. Tumor markers were unremarkable. A CT scan demonstrated no lymphadenopathy but identified a prominent left spermatic cord. Due to a suspicion of chronic torsion vs. malignancy, a left radical orchiectomy was performed. Pathology identified a hemorrhagic paratesticular venous malformation without signs of germ cell neoplasia, a rare entity.Keywords
While the classic presentation of testicular malignancy is a painless scrotal mass in greater than 90 percent of cases,1 pain associated with a scrotal mass does not rule out malignancy. The management of scrotal lesions concerning for malignancy includes radical orchiectomy regardless of negative tumor markers. In fact, of those presenting with acute scrotal pain with concern for testicular torsion, orchiectomy pathology samples have been reported to be positive for malignancy in 6.4% of patients in one small retrospective case series2 and 1.2% in a larger, multicenter study in adult acute testicular torsion.3
We report the presentation and management of 14-year-old who presented with an asymptomatic left testicular mass, identified following a resolved one-day episode of pain, with an ultrasound revealing a 4.5 cm avascular mass concerning for delayed testicular torsion vs. malignancy. Pathologic examination of the patient’s radical orchiectomy specimen revealed neither a chronic torsion nor malignancy, but rather, a paratesticular vascular malformation of a venous type that appeared to entirely replace the epididymis and with hemorrhagic necrosis identified within the testicular parenchyma.
A 14-year-old child presented to our pediatric urology practice with a left testicular mass. Approximately two weeks prior, he experienced gradual pain and discomfort in his left testicle and scrotum, albeit without accompanying abdominal pain, nausea, or vomiting. The discomfort persisted throughout the night but had resolved by the following morning. He noted that the testicle seemed different in size before the onset of pain. Since that single episode, he had been asymptomatic, reporting no hematuria, dysuria, fevers, weight loss, or back pain. During a subsequent visit to his primary care physician, a large, painless left testicular mass was identified, leading to a urology consultation.
Physical examination revealed a palpably normal right testis and a non-tender, painless left testis the size of a peach, with the scrotal skin firmly adherent to the inferior aspect of the testis. Scrotal ultrasound with Doppler revealed an enlarged, heterogeneous left testis with peripheral hyperemia but without internal blood flow (volume of 41 mL), raising concern for either a torsion with a late, non-viable presentation or a testicular malignancy (Figure 1). The right testis appeared normal and well-perfused (volume of 25.2 mL).
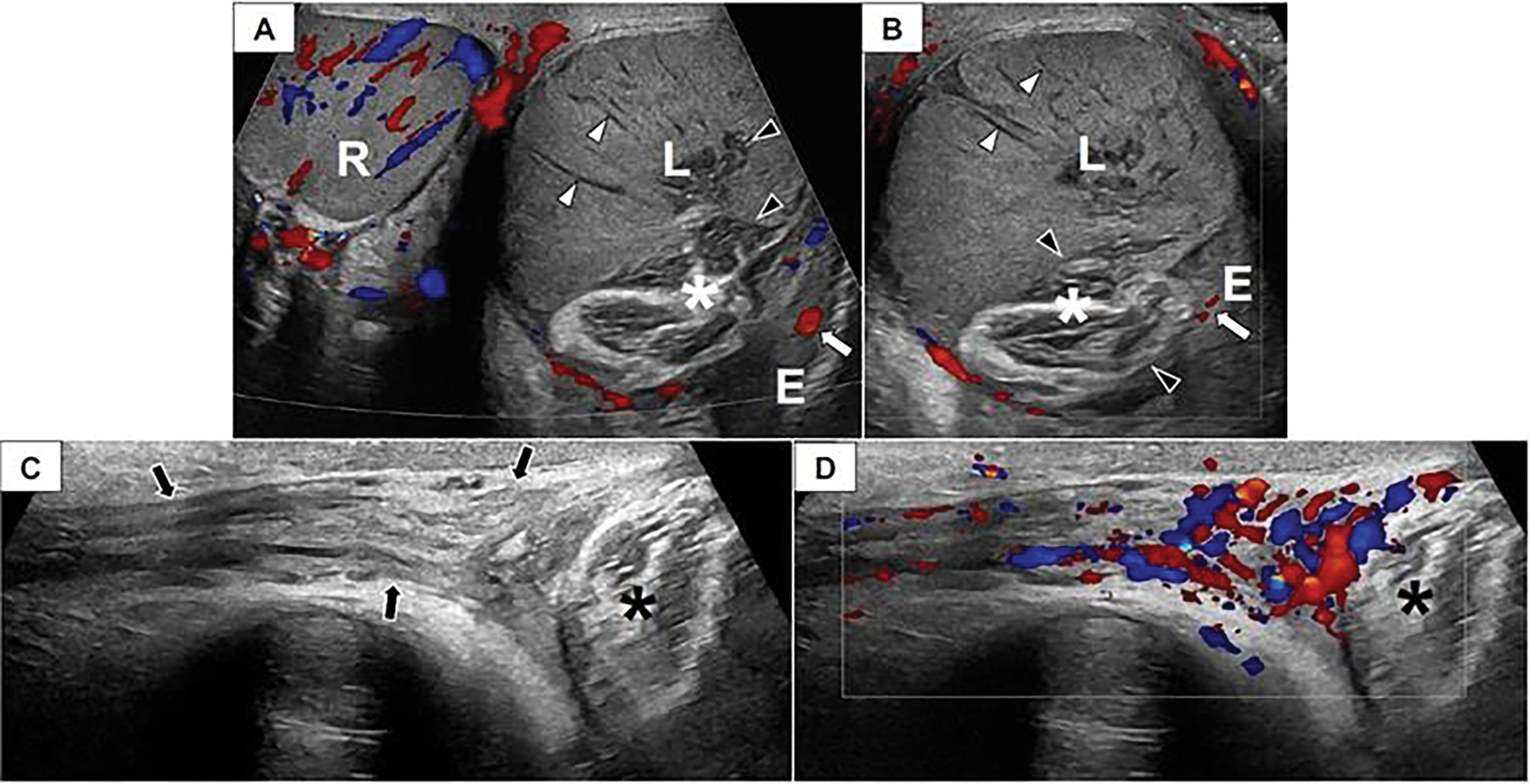
FIGURE 1. Scrotal doppler ultrasound. Paratesticular venous malformation in a 14-year-old male. Transverse color Doppler image of the scrotum (A) and longitudinal color Doppler image (B) of the left hemiscrotum depict a normal, well-perfused right (R) testis. The left (L) testis is enlarged and avascular with linear hypoechoic structures (white arrowheads) extending radially from the testicular periphery toward the mediastinum that may represent thrombosed vessels. There is a lobulated structure (asterisk) interposed between the epididymis (E) and the left (L) testis that consists of bundles of hypoechoic parallel linear lucencies surrounded by echogenic fibrofatty tissue. This structure appears to insinuate itself into the testicular mediastinum (black arrowheads). The epididymis has a normal appearance and demonstrates perfusion (white arrow). (C) A longitudinal grayscale image depicts a normal, non-twisted left spermatic cord (arrows) that appears well-perfused on (D) color Doppler evaluation. Asterisk, venous malformation.
Tumor markers were unremarkable (AFP 1.9, β-HCG < 0.6, and LDH 215). A CT scan of the chest, abdomen, and pelvis was negative for lymphadenopathy and showed a prominent left spermatic cord with partially visualized heterogeneous tissue at the superior aspect of the left scrotum.
The patient underwent left inguinal approach orchiectomy. Briefly, high ligation of the spermatic cord was performed via a subinguinal incision, as it was unclear if the testicle had malignant or benign pathology, and the orchiectomy was performed in the standard fashion.4 Pathologic analysis later revealed a dark red, poorly circumscribed, hemorrhagic paratesticular vascular malformation measuring 3.8 cm (Figure 2) as well as testicular parenchyma with extensive hemorrhagic necrosis (Figure 3A). The malformed vessels were predominantly venous in nature (Figure 3B–D) and the lesion appeared to entirely replace the epididymis while abutting, but not involving, the testicular hilum, tunica albuginea, or spermatic cord. Occasional thrombi were noted in the periphery of the lesion. There was no evidence of germ cell neoplasia or rhabdomyosarcoma.
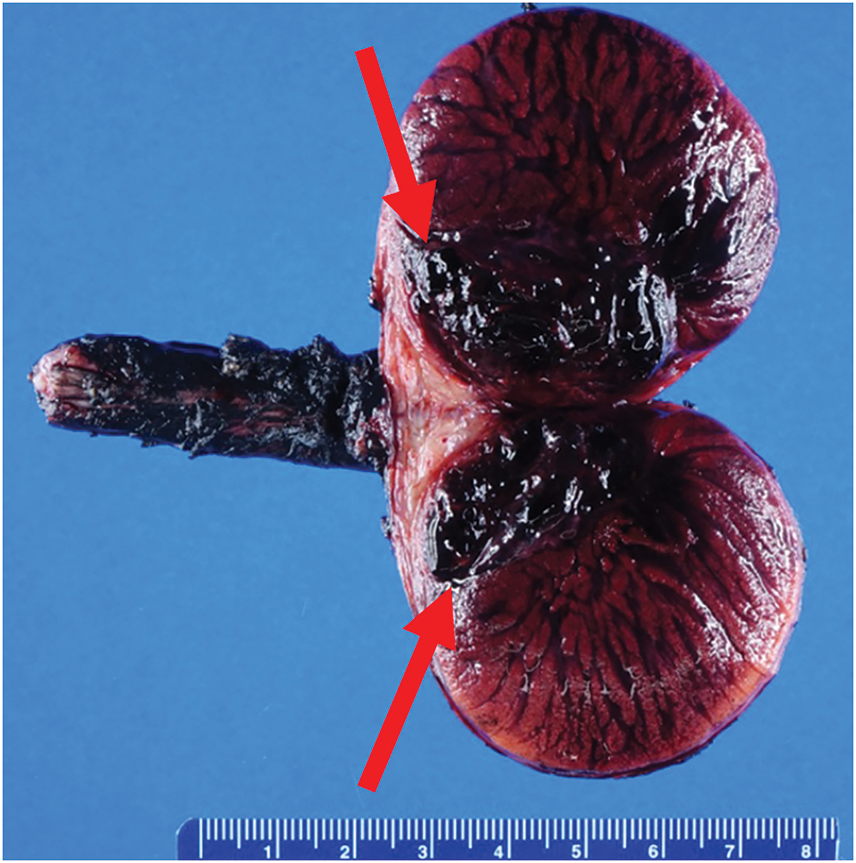
FIGURE 2. Gross pathologic analysis. A longitudinal cross section of the gross left orchiectomy specimen shows a 3.8 cm dark red, hemorrhagic paratesticular lesion (red arrows).
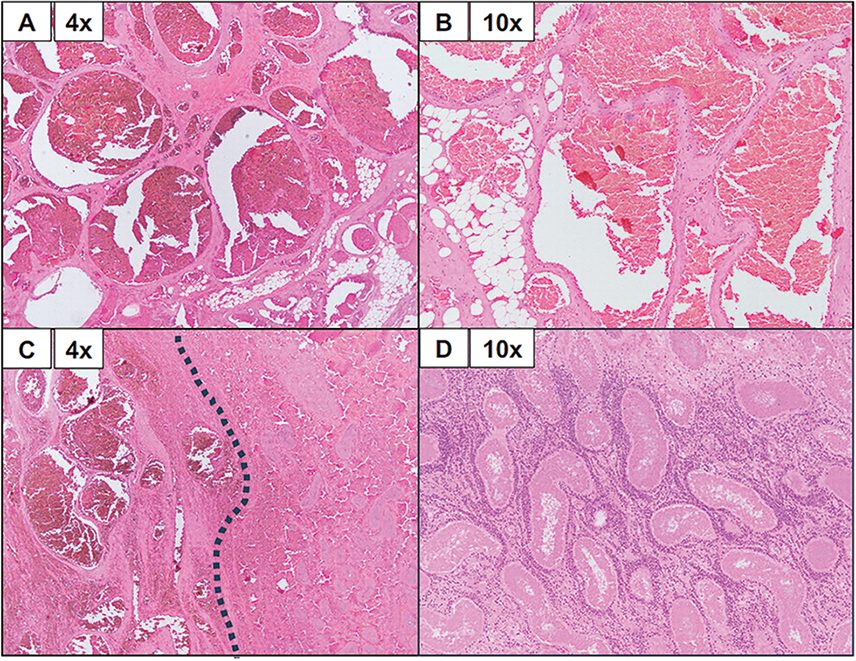
FIGURE 3. Histologic assessment of vascular malformation. A Representative histology of the testicular venous malformation using hematoxylin and eosin staining at 4× and 10× magnifications (specified in each frame). (A) Here, we can appreciate blood-filled, malformed vessels of a venous type. Malformed vessels are also noted in the adjacent fibroadipose tissue. In the absence of an arterial component, the findings are those of a venous malformation. (B) The malformed vessels are lined by bland-appearing endothelial cells. (C) This frame highlights the interface between venous malformation (left of the dotted line) and the adjacent hemorrhagic testicular parenchyma (right of the dotted line). (D) The testicular parenchyma shows complete necrosis of seminiferous tubules.
Vascular lesions in the testicle are exceedingly rare (having only been described in a handful of case reports) and can present with pain5 or be found incidentally (for example, during an infertility assessment.6 The first paratesticular arteriovenous malformation (AVM) was reported in a 20-year-old male with painful scrotal swelling after blunt trauma.7 This case differs from other previously reported case reports of paratesticular AVMs in that this was primarily a venous lesion without an arterial component, there was no vascular flow on Doppler ultrasound, and there was no flow to the ipsilateral testis. For this patient and others reported in the literature, intratesticular vascular malformations were an isolated finding and not associated with malformations in other organ systems.
Vascular malformations are a distinct entity from vascular tumors as malformations are secondary to inborn errors in vascular morphogenesis instead of true neoplasms. This case demonstrated an excess of irregularly arranged (malformed) vascular channels adjacent to the testicular parenchyma, and likely arose from the native paratesticular vascular channels. From this, pathologists then classify the malformation by type—venous, arteriovenous, or lymphatic.8 The pathology in this case is also markedly distinct from that of testicular torsion and hemangiomas. Torsion often demonstrates edema, congestion, and interstitial hemorrhage within the venous system, but does not demonstrate an increased number of vessels with an irregular arrangement. There are a limited number of case reports that describe infantile testicular hemangiomas as solid masses with prominent arterial and venous flow.9
In conclusion, this case highlights three important aspects in the management of testicular pathology. First, the initial assessment of a testicular mass includes a thorough history, physical examination, and color Doppler ultrasonography. For cases in which the diagnosis is unclear, newer contrast-enhanced ultrasound imaging techniques may be utilized to investigate a broader range of vascular and architectural abnormalities.10 Other groups have also utilized MRI to further characterize vascular lesions in the testicle. Second, the surgical management of a suspicious testicular lesion should be through an inguinal orchiectomy, as opposed to a scrotal approach, to avoid scrotal violation of lymphatic spread for potentially malignant masses. In pre-pubertal patients, where benign lesions are most common and testis-sparing surgery is encouraged, this should also be conducted via an inguinal approach. Third, the differential diagnoses for scrotal and testicular lesions should include vascular malformations.
Acknowledgement
The authors sincerely thank G. L. Ocampo for his assistance with this manuscript.
Funding Statement
VMR is funded by the AUA and Urology Care Foundation (Research Scholars Award) as well as an NIH NRSA training grant (5T32DK060442-20). HT is funded by the AUA and Urology Care Foundation (Research Scholars Award), SUFU (Chemodenervation Grant), and the Office of Faculty Development at Harvard Medical School.
Author Contributions
Conceptualization and supervision: H. Thaker. Writing: D. C. Leslie and V. M. Ramakrishnan. Editing: J . Putra, H. J. Paltiel, and H. Thaker. Pathology analysis: J . Putra. Radiologic analysis: H. J. Paltiel. Figure as-sembly: D. C. Leslie and V. M. Ramakrishnan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
Materials are available upon request.
Ethics Approval
Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Travis LB, Feldman DR, Fung C, Poynter JN, Lockley M, Frazier AL. Adolescent and young adult germ cell tumors: epidemiology, genomics, treatment, and survivorship. J Clin Oncol 2024;42(6):696–706. doi:10.1200/jco.23.01099. [Google Scholar] [CrossRef]
2. Uguz S, Yilmaz S, Guragac A, Topuz B, Aydur E. Association of torsion with testicular cancer: a retrospective study. Clin Genitourin Cancer 2016;14(1):e55–e57. doi:10.1016/j.clgc.2015.09.014. [Google Scholar] [CrossRef]
3. Pradere B, de Varennes AM, Benali NA et al. Torsion of the spermatic cord in adults: a multicenter experience in adults with surgical exploration for acute scrotal pain with suspected testicular torsion. Asian J Androl 2022;24(6):575–578. [Google Scholar]
4. Anderson E, Pascoe C, Sathianathen N, Katz D, Murphy D, Lawrentschuk N. Subinguinal orchiectomy—a minimally invasive approach to open surgery. BJUI Compass 2020;1(5):160–164. doi:10.1002/bco2.33. [Google Scholar] [CrossRef]
5. Sountoulides P, Bantis A, Asouhidou I, Aggelonidou H. Arteriovenous malformation of the spermatic cord as the cause of acute scrotal pain: a case report. J Med Case Rep 2007;1(1):110. doi:10.1186/1752-1947-1-110. [Google Scholar] [CrossRef]
6. Gulsen F, Mihmanli I, Kantarci F, Eren A, Ataus SO. Testicular arteriovenous malformation: gray-scale and color Doppler ultrasonography features. Case Rep Med 2011;2011(3):876206. doi:10.1155/2011/876206. [Google Scholar] [CrossRef]
7. Kang TW, Choi YD, Jeong YY et al. Intrascrotal extratesticular arteriovenous malformation. Urology 2004;64(3):590. doi:10.1016/j.urology.2004.04.040. [Google Scholar] [CrossRef]
8. Wassef M, Blei F, Adams D et al. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics 2015;136(1):e203–e214. doi:10.1542/peds.2014-3673. [Google Scholar] [CrossRef]
9. Laarif S, Trabelsi F, Daïb A, Ben Abdallah R, Hellal Y, Kaabar N. Inguinoscrotal hernia revealing a testicular hemangioma: report of a neonatal case. Urol Case Rep 2023;50(1):102491. doi:10.1016/j.eucr.2023.102491. [Google Scholar] [CrossRef]
10. Sridharan A, Hwang M, Kutty S et al. Translational research in pediatric contrast-enhanced ultrasound. Pediatr Radiol 2021;51(12):2425–2436. doi:10.1007/s00247-021-05095-8. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools